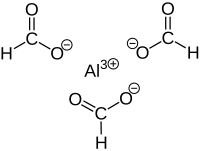
Aluminium formate is the aluminium salt of formic acid, with the chemical formula Al(HCOO)3. It can be produced via the reaction of aluminium soaps and formic acid.[1] Reaction between formic acid and aluminium hydroxide yields Al(HCOO)3(CO2)0.75(H2O)0.25(HCOOH)0.25. Upon activation at 180 °C, guest molecules are removed to obtain Al(HCOO)3.[2]
| |
| Names | |
|---|---|
| IUPAC name
Aluminium triformate
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.028.089 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C3H3AlO6 | |
| Molar mass | 162.033 g·mol−1 |
| Appearance | White powder |
| Insoluble | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
References
edit- ^ Oskar, Jochem. "ALUMINIUM FORMATE AND PROCESS OF MAKING THE SAME". United States Patent Office. Retrieved 2012-06-29.[dead link]
- ^ Evans H A, Mullangi D, Deng Z, et al. Aluminium formate, Al(HCOO)3: An earth-abundant, scalable, and highly selective material for CO2 capture. Science Advances, 2022, 8(44): eade1473. doi:10.1126/sciadv.ade1473
Further reading
edit- Hannelore, Rauh; Wilhelm Knoche (2010-06-10). "A kinetic study of the formation of aluminium formate in aqueous solution". Berichte der Bunsengesellschaft für physikalische Chemie. 83 (5): 518–521. doi:10.1002/bbpc.19790830513.
- Xue, Moxi; Gao, Baoyu; Li, Ruihua; Sun, Jianzhang (2018). "Aluminium formate (AF): Synthesis, characterization and application in dye wastewater treatment". Journal of Environmental Sciences. 74: 95–106. doi:10.1016/j.jes.2018.02.013. PMID 30340679. S2CID 53010618.