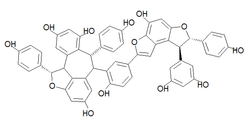
Amurensin K is an oligostilbene. It is a resveratrol tetramer found in Vitis amurensis.[1] Preliminary tests have shown it to be an effective neuraminidase inhibitor against the influenza A virus subtype H1N1.[2]
| |
| Names | |
|---|---|
| Preferred IUPAC name
(1S,6R,7S,11bS)-6-{5-[(7S,8S)-8-(3,5-Dihydroxyphenyl)-4-hydroxy-7-(4-hydroxyphenyl)-7,8-dihydrobenzo[1,2-b:4,3-b′]difuran-2-yl]-2-hydroxyphenyl}-1,7-bis(4-hydroxyphenyl)-1,6,7,11b-tetrahydrobenzo[6,7]cyclohepta[1,2,3-cd][1]benzofuran-4,8,10-triol | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C56H40O13 | |
| Molar mass | 920.91 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
References
edit- ^ Huang, Kai-Sheng; Lin, Mao; Cheng, Gui-Fang (September 2001). "Anti-inflammatory tetramers of resveratrol from the roots of Vitis amurensis and the conformations of the seven-membered ring in some oligostilbenes". Phytochemistry. 58 (2): 357–362. Bibcode:2001PChem..58..357H. doi:10.1016/S0031-9422(01)00224-2. PMID 11551564.
- ^ Nguyen, Thi Ngoc Anh; Dao, Trong Tuan; Tung, Bui Thanh; Choi, Hwanwon; Kim, Eunhee; Park, Junsoo; Lim, Seong-IL; Oh, Won Keun (2011). "Influenza A (H1N1) neuraminidase inhibitors from Vitis amurensis". Food Chemistry. 124 (2): 437–443. doi:10.1016/j.foodchem.2010.06.049. ISSN 0308-8146.