Artemether is a medication used for the treatment of malaria.[1][2] The injectable form is specifically used for severe malaria rather than quinine.[2] In adults, it may not be as effective as artesunate.[2] It is given by injection in a muscle.[2] It is also available by mouth in combination with lumefantrine, known as artemether/lumefantrine.[1][3]
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Many[1] |
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Intramuscular[2] Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| PDB ligand | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.189.847 |
| Chemical and physical data | |
| Formula | C16H26O5 |
| Molar mass | 298.379 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 86 to 88 °C (187 to 190 °F) |
| |
| |
| | |
Artemether causes relatively few side effects.[4] An irregular heartbeat may rarely occur.[4] While there is evidence that use during pregnancy may be harmful in animals, there is no evidence of concern in humans.[4] The World Health Organization (WHO) therefore recommends its use during pregnancy.[4] It is in the artemisinin class of medication.[4]
Artemether has been studied since at least 1981, and has been in medical use since 1987.[5] It is on the World Health Organization's List of Essential Medicines.[6]
Medical uses
editArtemether is an antimalarial drug for uncomplicated malaria caused by P. falciparum (and chloroquine-resistant P. falciparum) or chloroquine-resistant P. vivax parasites.[1][7] Artemether can also be used to treat severe malaria.[2]
The World Health Organization (WHO) recommends the treatment of uncomplicated P. falciparum with artemisinin-based combination therapy.[8] Given in combination with lumefantrine, it may be followed by a 14-day regimen of primaquine to prevent relapse of P. vivax or P. ovale malarial parasites and provide a complete cure.[9]
Artemether can also be used in treating and preventing trematode infections of schistosomiasis when used in combination with praziquantel.[10]
Artemether is rated category C by the FDA based on animal studies where artemisinin derivatives have shown an association with fetal loss and deformity. Some studies, however, do not show evidence of harm.[11][12]
Side effects
editPossible side effects include cardiac effects such as bradycardia and QT interval prolongation.[1][13] Also, possible central nervous system toxicity has been shown in animal studies.[14][15]
Drug interactions
editPlasma artemether level was found to be lower when the combination product was used with lopinavir/ritonavir.[15] There is also decreased drug exposure associated with concurrent use with efavirenz or nevirapine.[16][17]
Artemether/lumefantrine should not be used with drugs that inhibit CYP3A4.[1][18]
Hormonal contraceptives may not be as efficacious when used with artemether/lumefantrine.[18]
Pharmacology
editMechanism of action
editA possible mechanism of action is that artemisinin drugs exert their cidal action by inhibiting PfATP6. Since PfATP6 is an enzyme regulating cellular calcium concentration, its malfunctioning will lead to intracellular calcium accumulation, which in turns causes cell death.[19]
Pharmacokinetics
editAbsorption of artemether is improved 2- to 3-fold with food. It is highly bound to protein (95.4%). Peak concentrations of artemether are seen 2 hours after administration.[3]
Artemether is metabolized in the human body to the active metabolite, dihydroartemisinin, primarily by hepatic enzymes CYP3A4/5.[3] Both the parent drug and active metabolite are eliminated with a half-life of about 2 hours.[3]
Chemistry
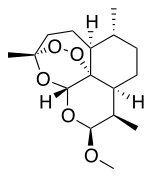
editArtemether is a methyl ether derivative of artemisinin, which is a peroxide-containing lactone isolated from the antimalarial plant Artemisia annua. It is also known as dihydroartemisinin methyl ether, but its correct chemical nomenclature is (+)-(3-alpha,5a-beta,6-beta,8a-beta, 9-alpha,12-beta,12aR)-decahydro-10-methoxy-3,6,9-trimethyl-3,12-epoxy-12H-pyrano(4,3-j)-1,2-benzodioxepin. It is a relatively lipophilic and unstable drug,[20] which acts by creating reactive free radicals in addition to affecting the membrane transport system of the plasmodium organism.[13]
References
edit- ^ a b c d e f "Artemether and Lumefantrine (Monograph)". Drugs.com. 22 February 2023. Retrieved 17 February 2024.
- ^ a b c d e f Esu EB, Effa EE, Opie ON, Meremikwu MM (June 2019). "Artemether for severe malaria". The Cochrane Database of Systematic Reviews. 6 (6): CD010678. doi:10.1002/14651858.CD010678.pub3. PMC 6580442. PMID 31210357.
- ^ a b c d "Coartem- artemether and lumefantrine tablet". DailyMed. 5 August 2019. Retrieved 26 April 2020.
- ^ a b c d e Kovacs SD, Rijken MJ, Stergachis A (February 2015). "Treating severe malaria in pregnancy: a review of the evidence". Drug Safety. 38 (2): 165–181. doi:10.1007/s40264-014-0261-9. PMC 4328128. PMID 25556421.
- ^ Rao Y, Zhang D, Li R (2016). Tu Youyou and the Discovery of Artemisinin: 2015 Nobel Laureate in Physiology or Medicine. World Scientific. p. 162. ISBN 9789813109919. Archived from the original on 2017-09-10.
- ^ World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. 2019. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ^ Makanga M, Krudsood S (October 2009). "The clinical efficacy of artemether/lumefantrine (Coartem)". Malaria Journal. 8 (Suppl 1): S5. doi:10.1186/1475-2875-8-S1-S5. PMC 2760240. PMID 19818172.
- ^ Treatment of Uncomplicated Plasmodium falciparum Malaria. World Health Organization. 2015-01-01. Archived from the original on 2017-09-10.
- ^ Treatment Of Uncomplicated Malaria Caused By P. vivax, P. ovale, P. malariae or P. knowlesi. World Health Organization. 2015-01-01. Archived from the original on 2017-09-10.
- ^ Pérez del Villar L, Burguillo FJ, López-Abán J, Muro A (2012-01-01). "Systematic review and meta-analysis of artemisinin based therapies for the treatment and prevention of schistosomiasis". PLOS ONE. 7 (9): e45867. Bibcode:2012PLoSO...745867P. doi:10.1371/journal.pone.0045867. PMC 3448694. PMID 23029285.
- ^ Dellicour S, Hall S, Chandramohan D, Greenwood B (February 2007). "The safety of artemisinins during pregnancy: a pressing question". Malaria Journal. 6: 15. doi:10.1186/1475-2875-6-15. PMC 1802871. PMID 17300719.
- ^ Piola P, Nabasumba C, Turyakira E, Dhorda M, Lindegardh N, Nyehangane D, et al. (November 2010). "Efficacy and safety of artemether-lumefantrine compared with quinine in pregnant women with uncomplicated Plasmodium falciparum malaria: an open-label, randomised, non-inferiority trial". The Lancet. Infectious Diseases. 10 (11): 762–769. doi:10.1016/S1473-3099(10)70202-4. hdl:10144/116337. PMID 20932805.
- ^ a b "Artemether". www.antimicrobe.org. Archived from the original on 2017-02-23. Retrieved 2016-11-09.
- ^ "WHO Model Prescribing Information: Drugs Used in Parasitic Diseases - Second Edition: Protozoa: Malaria: Artemether". apps.who.int. Archived from the original on 2016-11-10. Retrieved 2016-11-09.
- ^ a b Askling HH, Bruneel F, Burchard G, Castelli F, Chiodini PL, Grobusch MP, et al. (September 2012). "Management of imported malaria in Europe". Malaria Journal. 11: 328. doi:10.1186/1475-2875-11-328. PMC 3489857. PMID 22985344.
- ^ Van Geertruyden JP (April 2014). "Interactions between malaria and human immunodeficiency virus anno 2014". Clinical Microbiology and Infection. 20 (4): 278–285. doi:10.1111/1469-0691.12597. PMC 4368411. PMID 24528518.
- ^ Kiang TK, Wilby KJ, Ensom MH (February 2014). "Clinical pharmacokinetic drug interactions associated with artemisinin derivatives and HIV-antivirals". Clinical Pharmacokinetics. 53 (2): 141–153. doi:10.1007/s40262-013-0110-5. PMID 24158666. S2CID 1281113.
- ^ a b Stover KR, King ST, Robinson J (April 2012). "Artemether-lumefantrine: an option for malaria". The Annals of Pharmacotherapy. 46 (4): 567–577. doi:10.1345/aph.1Q539. PMID 22496476. S2CID 7678606.
- ^ Guo Z (March 2016). "Artemisinin anti-malarial drugs in China". Acta Pharmaceutica Sinica. B. 6 (2): 115–124. doi:10.1016/j.apsb.2016.01.008. PMC 4788711. PMID 27006895.
- ^ De Spiegeleer BM, D'Hondt M, Vangheluwe E, Vandercruyssen K, De Spiegeleer BV, Jansen H, et al. (November 2012). "Relative response factor determination of β-artemether degradants by a dry heat stress approach". Journal of Pharmaceutical and Biomedical Analysis. 70: 111–116. doi:10.1016/j.jpba.2012.06.002. hdl:1854/LU-2938963. PMID 22770733.