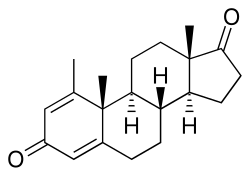
Atamestane (developmental code name SH-489), also known as metandroden, as well as 1-methylandrosta-1,4-diene-3,17-dione, is a steroidal aromatase inhibitor that was studied in the treatment of cancer.[2] It blocks the production of estrogen in the body. The drug is selective, competitive, and irreversible in its inhibition of aromatase.[3][additional citation(s) needed]
 | |
| Clinical data | |
|---|---|
| Other names | SH-489; Metandroden; 1-Methylandrosta-1,4-diene-3,17-dione |
| Routes of administration | By mouth[1] |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C20H26O2 |
| Molar mass | 298.426 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Synthesis
editReaction of the known compound, androstadienedione, (1) with Gilman reagent followed by acetylation with acetic anhydride gives the enol acetate (2). Bromination with 1,3-dibromo-5,5-dimethylhydantoin gives an intermediate (3) which on treatment with magnesium oxide yields atamestane (4).[4] Alternatively the steroid (5) can be oxidized with benzeneselenol, or the natural product, boldenone (6) can be oxidized with a mixture of chromium trioxide and sulfuric acid.[5]
References
edit- ^ el Etreby MF, Nishino Y, Habenicht UF, Henderson D (1991-11-12). "Atamestane, a new aromatase inhibitor for the management of benign prostatic hyperplasia". Journal of Andrology. 12 (6): 403–414. doi:10.1002/j.1939-4640.1991.tb00283.x. PMID 1722797.
- ^ Elks J (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 794–. ISBN 978-1-4757-2085-3.
- ^ el Etreby MF (March 1993). "Atamestane: an aromatase inhibitor for the treatment of benign prostatic hyperplasia. A short review". The Journal of Steroid Biochemistry and Molecular Biology. 44 (4–6): 565–72. doi:10.1016/0960-0760(93)90260-4. PMID 7682838. S2CID 53256276.
- ^ US patent 4871482, Klaus N, Hanfried A, "Process for the preparation of 1-methylandrosta-1,4-diene-3,17,dione, and the novel intermediates for this process", issued 1989-10-03, assigned to Schering AG
- ^ Künzer H, Sauer G, Wiechert R (1989). "Regioselective synthesis of ring a polymethylated steroids in the androstane series". Tetrahedron. 45 (20): 6409–6426. doi:10.1016/S0040-4020(01)89518-1.
External links
edit- Atamestane entry in the public domain NCI Dictionary of Cancer Terms
This article incorporates public domain material from Dictionary of Cancer Terms. U.S. National Cancer Institute.