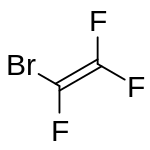
Bromotrifluoroethylene (BTFE) is a halogenated ethylene derivative with the chemical formula F2CCBrF. It is a highly flammable colourless gas with a musty odour resembling phosgene. It can polymerise spontaneously.[3]
| |
| Names | |
|---|---|
| IUPAC name
bromotrifluoroethene
| |
| Other names
trifluorovinyl bromide, trifluorobromoethylene
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.009.045 |
| EC Number |
|
PubChem CID
|
|
| UN number | 2419 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C2BrF3 | |
| Molar mass | 160.921 g·mol−1 |
| Appearance | colourless gas |
| Odor | moldy,[1] phosgene-like[2] |
| Boiling point | –2.5°C[2] |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
spontaneous polymerisation, flammable[3] |
| GHS labelling:[1] | |
   
| |
| Danger | |
| H220, H280, H315, H319, H330, H332, H335 | |
| P203, P210, P222, P260, P261, P264, P264+P265, P271, P280, P284, P302+P352, P304+P340, P305+P351+P338, P316, P317, P319, P320, P321, P332+P317, P337+P317, P362+P364, P377, P381, P403, P403+P233, P405, P410+P403, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Preparation
editBromotrifluoroethylene can be prepared from chlorotrifluoroethylene with high yields:[3][4]
- F2C=ClF + HBr → CF2BrCHClF + Zn → CF2=CHF + ZnBrCl
- CF2=CHF + Br2 → CF2BrCHBrF + KOH → CF2CHBr + KBr + H2O
It was first prepared by the Belgian chemist Frédéric Swarts in 1899.[5]
Reactions and uses
editBromotrifluoroethylene forms metal complexes with substituted phosphine compounds and platinum(II).[6]
BTFE can polymerise on standing. Spontaneous polymerisation may be inhibited by addition of tributylamine.[4] UV light and heat may accelerate polymerisation.[4] It participates in various co-polymerisation reactions.[2] BTFE telomers are oily liquids sold under the tradename BFC oil. The telomers can be prepared with fluorotrichloromethane or tetrachloromethane as telogens. If tetrachloromethane is used for the telomerisation, it will have -CCl3 terminals.[5] It is a useful reagent for the synthesis of trifluorovinyl compounds.[3]
References
edit- ^ Hazardous Substance Fact Sheet, New Jersey Department of Health PDF
- ^ a b c Yaws, C. L., Braker, W. (2001). Matheson gas data book. Page 69
- ^ a b c d Arthur J. Elliott, Bromotrifluoroethylene in Kirk-Othmer Encyclopedia of Chemical Technology
- ^ a b c Fluorine chemistry: a comprehensive treatment (1995), pages 479–480
- ^ a b Industrial Polymers and Radiation: Proceedings of the Symposium Held at Sardar Patel University, Vallabh Vidyanagar, Gujarat, February 12–14, 1979.
- ^ V.A. Mukhedkar, B.J. Kavathekar, A.J. Mukhedkar, Reactions of metal complexes Rearrangement reactions of bromotrifluoroethylene-bis(substituted phosphine)-platinum (II), Journal of Inorganic and Nuclear Chemistry, Volume 37, Issue 2, February 1975, Pages 483-485