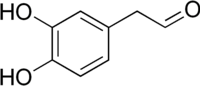
The catecholaldehyde hypothesis is a scientific theory positing that neurotoxic aldehyde metabolites of the catecholamine neurotransmitters dopamine and norepinephrine are responsible for neurodegenerative diseases involving loss of catecholaminergic neurons, for instance Parkinson's disease.[1][2] The specific metabolites thought to be involved include 3,4-dihydroxyphenylacetaldehyde (DOPAL) and 3,4-dihydroxyphenylglycolaldehyde (DOPEGAL), which are formed from dopamine and norepinephrine by monoamine oxidase, respectively.[1][2] These metabolites are subsequently inactivated and detoxified by aldehyde dehydrogenase (ALDH).[1][2] DOPAL and DOPEGAL are monoaminergic neurotoxins in preclinical models and inhibition of and polymorphisms in ALDH are associated with Parkinson's disease.[1][2][3][4] The catecholaldehyde hypothesis additionally posits that DOPAL oligomerizes with α-synuclein resulting in accumulation of oligomerized α-synuclein (i.e., synucleinopathy) and that this contributes to cytotoxicity.[1][2][5][3]
See also
editReferences
edit- ^ a b c d e Goldstein DS (February 2020). "The catecholaldehyde hypothesis: where MAO fits in". J Neural Transm (Vienna). 127 (2): 169–177. doi:10.1007/s00702-019-02106-9. PMC 10680281. PMID 31807952.
- ^ a b c d e Goldstein DS (June 2021). "The Catecholaldehyde Hypothesis for the Pathogenesis of Catecholaminergic Neurodegeneration: What We Know and What We Do Not Know". Int J Mol Sci. 22 (11): 5999. doi:10.3390/ijms22115999. PMC 8199574. PMID 34206133.
- ^ a b Goldstein DS, Sharabi Y (January 2019). "The heart of PD: Lewy body diseases as neurocardiologic disorders". Brain Res. 1702: 74–84. doi:10.1016/j.brainres.2017.09.033. PMC 10712237. PMID 29030055.
- ^ Marchitti SA, Deitrich RA, Vasiliou V (June 2007). "Neurotoxicity and metabolism of the catecholamine-derived 3,4-dihydroxyphenylacetaldehyde and 3,4-dihydroxyphenylglycolaldehyde: the role of aldehyde dehydrogenase". Pharmacol Rev. 59 (2): 125–150. doi:10.1124/pr.59.2.1. PMC 2647328. PMID 17379813.
- ^ Goldstein DS, Kopin IJ, Sharabi Y (December 2014). "Catecholamine autotoxicity. Implications for pharmacology and therapeutics of Parkinson disease and related disorders". Pharmacol Ther. 144 (3): 268–282. doi:10.1016/j.pharmthera.2014.06.006. PMC 4591072. PMID 24945828.