Chromium(III) phosphate describes inorganic compounds with the chemical formula CrPO4·(H2O)n, where n = 0, 4, or 6. All are deeply colored solids. Anhydrous CrPO4 is green. The hexahydrate CrPO4·6H2O is violet.
| |
| Names | |
|---|---|
| IUPAC name
Chromium(III) phosphate
| |
| Other names
Chromium phosphate, Chromium monophosphate, Chromium orthophosphate, Chromic phosphate
| |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.029.219 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| CrPO4 | |
| Molar mass | 146.97 g/mol |
| Density | 4.236 g/cm3[1] |
| Melting point | 1,907 °C (3,465 °F; 2,180 K)[1] |
| Boiling point | 2,671 °C (4,840 °F; 2,944 K) |
| insoluble, exothermal blue solution[1] | |
| Structure | |
| monoclinic[1] | |
| Hazards | |
| NIOSH (US health exposure limits): | |
PEL (Permissible)
|
TWA 1 mg/m3[2] |
REL (Recommended)
|
TWA 0.5 mg/m3[2] |
IDLH (Immediate danger)
|
250 mg/m3[2] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Synthesis
editChromium phosphate is prepared by treating a phosphoric acid solution of chromium(VI) oxide with hydrazine.[1]
Hexahydrated chromium(III) phosphate
editHexahydrate chromium phosphate, CrPO4·6H2O, is prepared by reducing chromium trioxide, CrO3, with ethanol in the presence of orthophosphoric acid, H3PO4, at temperatures ranging from −24 °C to +80 °C.[3]
Mesoporous phase
editGel-like chromium(III) phosphate is prepared through the reduction of ammonium dichromate, (NH4)2Cr2O7, using ethanol, CH3COOH, and nitric acid, HNO3. This process is done in the presence of ammonium dihydrogen phosphate and urea at an elevated temperature where tetradecyltrimethylammonium bromide (TTBr) is used as structure directing agent.[4]
Films
editPreparation of textured chromium phosphate is carried out by mixing equimolar solutions of aqueous chromium nitrate and diammonium phosphate in a dish placed in a sealed chamber with the low temperature ammonia vapor catalyst diffusing into the solution at a constant rate. After 24h, the resulting purple film grows out from the liquid through the hydrolysis and polycondensation occurring in the reaction environment at the air/liquid and film/liquid boundary. Surface tension makes the film compact making it easy to insert a microscope slide and lift the film from underneath the solution surface. Once obtained the solution is washed with deionized water and ethanol, then dried in a vacuum.[5]
Amorphous phase
editThe preparation of anhydrous chromium(III) phosphate begins by grinding a mixture of 75 mol% of chromium(III) oxide, Cr2O3, and 25 mol% of pure ammonium hydrogen phosphate, (NH4)2HPO4. This mixture is pressed into pellets and heated under air pressure at 400 °C for 24h in order to remove ammonia and water. After this, a heating sequence of 450 °C (24 h), 700 °C (3⋅24 h), 800 °C (24 h) and 850 °C (2⋅24 h) occurs. The pellet mixture is gradually cooled thereafter.[6]
Physical properties
editCrystal structure
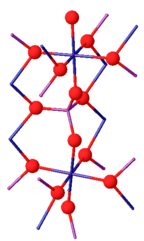
editChromium(III) phosphate can exist as two isomorphs. Its β-isoform is orthorhombic with the Cmcm space group (a = 0.5165, b = 0.7750, c = 0.6131 nm). The structure consists of infinite chains of trans edge-sharing CrO6 octahedra, which run parallel to the c-axis, and are linked by PO4 tetrahedra. Above 1175 °C, β-CrPO4 converts to α-CrPO4. α-CrPO4 is orthorhombic as well, with the Imma space group (a = 1.0380, b = 1.2845, c = 0.6278 nm). The structure consists of an infinite network of linked polyhedra with a CrO6 octahedron and a PO4 tetrahedron sharing a common edge. The Cr3+ site form edge-sharing Cr(2)/Cr(2') pairs and share two corners with the four Cr(1) octahedra.[7]
Magnetic properties
editThe magnetic properties of the β-CrPO4 are a result of the cation-cation distances along the octahedral chains which give rise to strong direct-exchange interactions and even metal-metal bonding. Neutron diffraction studies reveal that the spiral moments in β-CrPO4 are collinear and anti-ferromagnetically coupled along the chains in the 001 planes, at low temperature (5K, μeff = 2.55 μB).[7] Observations from a diffraction study has shown that at low temperature(2K), the α-CrPO4 octahedra CrO6 units build up an infinite, three-dimensional network expected to provide strong Cr-O-Cr magnetic superexchange linkages with exchange pathway through the phosphate group. These linkages give the structure its anti-ferromagnetic characteristic (Ɵ = -35.1 K, μeff = 3.50 μB) which results in the anti-parallel magnetic spins in the plane that is perpendicular to the chains of the octahedral CrO6.[8]
Chemical properties
editIon exchange
editAt a high temperature and pH ranging from 283-383K and pH 4-7 respectively, equilibrated KOH/HCl solution, insoluble CrPO4 solid and aqueous cation solution yield a sorption reaction. Studies reveal that CrPO4 catalyzes the adsorption of divalent cations onto its amorphous surface through the cation exchange mechanism. The mechanism suggests that the H+ ions are liberated from the solid to aqueous phase as the cations become hydrolyzed and adsorb onto the catalyst surface. Thus, a decrease in the pH of the reaction is used as a direct indicator of the rate of adsorption in the reaction:
A plot of the Kurbatov equation is used to relate the release of H+ ion to the equilibrium constant of the reaction:
where Kd (l g-1) represents the distribution coefficient, and n is the slope of the straight line giving an indication of the H+/Mz+ stoichiometry of the exchange reaction. Under similar conditions, the selectivity of CrPO4 for dative cations follows the sequence: Pb2+ > Cu2+ > Ni2+ ≅ Cd2+. Increases in temperature and pH enhances the ion exchange reaction.[9]
Chromium(III) phosphate is also used to catalyze cation exchange in sorption reactions. This catalysis is widely used in the reduction of metal toxicity during environmental clean-ups. This has been applied in decreasing the concentration of lead in aquatic habitat and drinking water.[9]
Application
editAnti-corrosive coating
editPaints containing chromium(III) phosphate have been used as corrosion resistant coating for metals. The paints consist of aqueous acidic chromium (III) phosphate solution which convert to a consistent film when applied onto metals heavily used in manufacturing and utility such as zinc, zinc alloy, aluminum and aluminum alloy substrates. Application methods include electroplating, immersing or spraying the solution on the surface of the substrate.[10]
Catalyst
editChromium (III) phosphate has various applications in the polymer industry. Combined chromium(III) aluminum phosphate is widely used as a catalyst in the alkylation of aromatic hydrocarbons using alcohols such as the methylation of toluene using methanol. The alcohol is dehydrated into ether while the alkyl substituted product could be used as an intermediate in the manufacture of synthetic fibers such as poly(ethylene terephthalate).[11]
Polymer
editPretreatment with chromium (III) phosphate-silicate is also used as a laminated structure to dampen vibration and noise in a motor.[12]
Toxicity
editAlthough chromium(III) phosphate is hardly soluble in water, overexposure to the compound from the environment, industrial location and abrasions from metal on metal implants could have harmful effects. The toxicity of chromium(III) phosphate depends on the duration of exposure, chromium(III) phosphate concentration, entry routes across a membrane barrier and release of trivalent chromium ion from the chromium(III) phosphate. Macrophage cells in the body exposed to chromium(III) phosphate engulf or phagocytize the compound into its endosomal and lysosomal environment which is acidic. This catalyzes a proteolytic reaction yielding a dose-dependent increase in chromium(III) ion release in the affected cells. The Cr3+ ions has toxic effects on the proteins of the cytosol and mitochondria by oxidatively modifying their chemical properties thus disenabling from performing their functions. Proteins with high metal affinity such as enolase, catalase enzymes and hemoglobin, ferritin molecular transporters are affected. This may ultimately lead to nephrotoxicity, reproductive and developmental toxicity due to tissue damage, necrosis and inflammation.[13]
Further reading
edit- Mustafa, S.; Murtaza, S.; Naeem, A.; Farina, K. (2010). "Ion Exchange Sorption Of Pb2+ Ions On CrPO4". Environmental Technology. 26 (4): 353–359. doi:10.1080/09593332608618544. PMID 15906486. S2CID 30688737.
See also
editExternal links
editReferences
edit- ^ a b c d e Brauer, Georg (1965) [1962]. Handbuch Der Präparativen Anorganischen Chemie [Handbook of Preparative Inorganic Chemistry] (in German). Vol. 2. Stuttgart; New York, New York: Ferdinand Enke Verlag; Academic Press, Inc. p. 1341. ISBN 978-0-32316129-9. Retrieved 2014-01-10.
- ^ a b c NIOSH Pocket Guide to Chemical Hazards. "#0141". National Institute for Occupational Safety and Health (NIOSH).
- ^ Vasović, Dušanka D.; Stojaković, Djordje R. (1989). "Preparation and properties of some amorphous chromium(III) phosphates". Journal of Non-Crystalline Solids. 109 (1). Elsevier BV: 129–132. Bibcode:1989JNCS..109..129V. doi:10.1016/0022-3093(89)90451-1. ISSN 0022-3093.
- ^ Tarafdar, A.; Biswas, Susanta; Pramanik, N.K.; Pramanik, P. (2006). "Synthesis of mesoporous chromium phosphate through an unconventional sol–gel route". Microporous and Mesoporous Materials. 89 (1–3). Elsevier BV: 204–208. doi:10.1016/j.micromeso.2005.10.027. ISSN 1387-1811.
- ^ Gomm, John R.; Schwenzer, Birgit; Morse, Daniel E. (2007). "Textured films of chromium phosphate synthesized by low-temperature vapor diffusion catalysis". Solid State Sciences. 9 (5). Elsevier BV: 429–431. Bibcode:2007SSSci...9..429G. doi:10.1016/j.solidstatesciences.2007.03.012. ISSN 1293-2558.
- ^ Bosacka, M.; Jakubus, P.; Rychowska-Himmel, I. (2007). "Obtaining Of Chromium(III) Phosphates(V) In The Solid-State And Their Thermal Stability". Journal of Thermal Analysis and Calorimetry. 88 (1): 133–137. doi:10.1007/s10973-006-8050-z. ISSN 1388-6150. S2CID 98435405.
- ^ a b Attfield, J. Paul; Battle, Peter D.; Cheetham, Anthony K.; Johnson, David C. (1989). "Magnetic structures and properties of .alpha.-chromium phosphate and .alpha.-chromium arsenate". Inorganic Chemistry. 28 (7). American Chemical Society (ACS): 1207–1213. doi:10.1021/ic00306a004. ISSN 0020-1669.
- ^ Attfield, J. Paul; Battle, Peter D.; Cheetham, Anthony K. (1985). "The spiral magnetic structure of β-chromium(III) orthophosphate (β-CrPO4)". Journal of Solid State Chemistry. 57 (3). Elsevier BV: 357–361. Bibcode:1985JSSCh..57..357A. doi:10.1016/0022-4596(85)90199-9. ISSN 0022-4596.
- ^ a b Mustafa, S.; Murtaza, S.; Naeem, A.; Farina, K. (2010). "Ion Exchange Sorption Of Pb2+ Ions On CrPO4". Environmental Technology. 26 (4): 353–359. doi:10.1080/09593332608618544. PMID 15906486. S2CID 30688737.
- ^ US patent 20070243397, Ludwig, R. & Recker, A., "Chromium(VI)-free, aqueous acidic Chromium(III) conversion solutions", published 2007, assigned to Columbia Chemical Corporation, Ohio
- ^ US patent 4543436, Johnson, M.M. & Nowack, G.P., "Chromium phosphate as an alkylation catalyst", published September 24, 1985
- ^ US patent 20090252989, Swanson, R. & Hufnagel, A., "Laminated Viscoelastic Damping Structure and Method of making the same", published October 8, 2009
- ^ Scharf, B.; Clement, C.C.; Zolla, V.; Perino, G.; Yan, B.; Elci, S.G.; Purdue, E.; Goldring, S.; Macaluso, F.; Cobelli, N; Vachet, R.W; Santambrogio, L. (2015). "Molecular Analysis of Chromium and Cobalt-related toxicity". Scientific Reports. 2014: 5729. doi:10.1038/srep05729. PMC 4103093. PMID 25034144.