Conopharyngine is the major alkaloid present in the leaves and stem-bark of Tabernaemontana pachysiphon and Conopharyngia durissima.[1][2][3] It is closely related voacangine and coronaridine. Conopharyngine pseudoindoxyl, a derivative of it, is also found in the same plant Tabernaemontana pachysiphon.[4]
| |
| Names | |
|---|---|
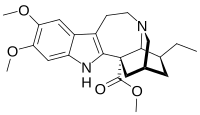
| IUPAC name
Methyl (1S,15S,17S,18S)-17-ethyl-6,7-dimethoxy-3,13-diazapentacyclo[13.3.1.02,10.04,9.013,18]nonadeca-2(10),4,6,8-tetraene-1-carboxylate
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| C23H30N2O4 | |
| Molar mass | 398.503 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Pharmacology
editIt possess central nervous system stimulant activity and produces bradycardia and hypotension in cats. It has weak acetylcholinesterase inhibitory activity and significantly increases hexobarbitone induced sleeping time.[5]
Toxicity
editIt has low intravenous toxicity in mice (LD50 = 143 mg/kg).[5]
See also
editReferences
edit- ^ "Tabernaemontana pachysiphon".
- ^ van Beek TA, de Smidt C, Verpoorte R (1985). "Phytochemical investigation of Tabernaemontana crassa". Journal of Ethnopharmacology. 14 (2–3): 315–8. doi:10.1016/0378-8741(85)90096-0. PMID 4094474.
- ^ Renner, U.; Prins, D. A.; Stoll, W. G. (1959). "Alkaloide ausConopharyngia durissima STAPF Isovoacangin, Conopharyngin, Conodurin und Conoduramin". Helvetica Chimica Acta. 42 (5): 1572–1581. doi:10.1002/hlca.19590420519. ISSN 0018-019X.
- ^ Crooks PA, Robinson B (October 1973). "Conopharyngine pseudoindoxyl, a new alkaloid from Tabernamontana pachysiphon Stapf. var cumminsii (Stapf.) H. Huber". The Journal of Pharmacy and Pharmacology. 25 (10): 820–3. doi:10.1111/j.2042-7158.1973.tb09948.x. PMID 4149744. S2CID 9261649.
- ^ a b Carroll PR, Starmer GA (May 1967). "Studies on the pharmacology of conopharyngine, an indole alkaloid of the voacanga series". British Journal of Pharmacology and Chemotherapy. 30 (1): 173–85. doi:10.1111/j.1476-5381.1967.tb02123.x. PMC 1557242. PMID 6039971.