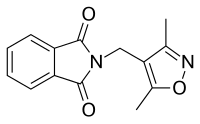
DIMP (developmental code name Ro 7-8117), or N-(3,5-dimethyl-4-isoxazolylmethyl)phthalimide, is a nonsteroidal antiandrogen (NSAA) structurally related to thalidomide (which also binds to and antagonizes the androgen receptor (AR))[1][2][3] that was first described in 1973 and was never marketed.[4] Along with flutamide, it was one of the earliest NSAAs to be discovered,[5] and for this reason, has been described as a "classical" NSAA.[1][2][3] The drug is a selective, competitive, silent antagonist of the AR,[4][6] although it is described as an "only relatively weak competitor".[7][8] Its relative binding affinity for the androgen receptor is about 2.6% of that of metribolone.[9] DIMP possesses no androgenic, estrogenic, progestogenic, or antigonadotropic activity,[4] but it does reverse the antigonadotropic effects of testosterone, indicating that, like other pure AR antagonists, it is progonadotropic.[4]
 | |
| Clinical data | |
|---|---|
| Other names | Ro 7-8117; N-(3,5-Dimethyl-4-isoxazolylmethyl)phthalimide |
| Drug class | Nonsteroidal antiandrogen |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C14H12N2O3 |
| Molar mass | 256.261 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
DIMP is the lead antiandrogen of the phthalimide group of nonsteroidal AR ligands, and a variety of AR ligands with higher affinity for the AR have been derived from DIMP and thalidomide.[10][11]
See also
editReferences
edit- ^ a b Hashimoto Y, Tanatani A, Nagasawa K, Miyachi H (2004). "Thalidomide as a multitarget drug and its application as a template for drug design". Drugs of the Future. 29 (4): 383. doi:10.1358/dof.2004.029.04.792298. ISSN 0377-8282.
- ^ a b Liu B, Su L, Geng J, Liu J, Zhao G (October 2010). "Developments in nonsteroidal antiandrogens targeting the androgen receptor". ChemMedChem. 5 (10): 1651–1661. doi:10.1002/cmdc.201000259. PMID 20853390. S2CID 23228778.
- ^ a b Hashimoto Y (July 2003). "Structural development of synthetic retinoids and thalidomide-related molecules". Cancer Chemotherapy and Pharmacology. 52 (Suppl 1): S16–S23. doi:10.1007/s00280-003-0590-3. PMID 12819930. S2CID 22663471.
- ^ a b c d Boris A, Scott JW, DeMartino L, Cox DC (March 1973). "Endocrine profile of a nonsteroidal antiandrogen N-(3,5-dimethyl-4-isoxazolylmethyl)phthalimide (DIMP)". Acta Endocrinologica. 72 (3): 604–614. doi:10.1530/acta.0.0720604. PMID 4739363.
- ^ Singhal RL, Thomas JA (1 January 1976). Cellular Mechanisms Modulating Gonadal Action. University Park Press. p. 239. ISBN 978-0-8391-0776-7.
- ^ Ahlin K, Forsberg JG, Jacobsohn D, Thore-Berger B (1975). "The male genital tract and the nipples of male and female offspring of rats given the non-steroidal antiandrogens DIMP and Sch 13521, during pregnancy". Archives d'Anatomie Microscopique et de Morphologie Expérimentale. 64 (1): 27–44. PMID 1217898.
- ^ Heyns W, Verhoeven G, De Moor P (May 1976). "Androgen binding in rat uterus cytosol. Study of the specificity". Journal of Steroid Biochemistry. 7 (5): 335–343. doi:10.1016/0022-4731(76)90092-3. PMID 180344.
- ^ Rasmusson GH (January 1986). "Chemical control of androgen action.". In Daily PM, Pawsen PA (eds.). Annual Reports in Medicinal Chemistry. Vol. 21. Academic Press. pp. 179-188 (182). ISBN 978-0-08-058365-5.
- ^ Wakeling AE, Furr BJ, Glen AT, Hughes LR (December 1981). "Receptor binding and biological activity of steroidal and nonsteroidal antiandrogens". Journal of Steroid Biochemistry. 15: 355–359. doi:10.1016/0022-4731(81)90297-1. PMID 7339263.
- ^ Gao W, Bohl CE, Dalton JT (September 2005). "Chemistry and structural biology of androgen receptor". Chemical Reviews. 105 (9): 3352–3370. doi:10.1021/cr020456u. PMC 2096617. PMID 16159155.
- ^ Kaur P, Khatik GL (2016). "Advancements in Non-steroidal Antiandrogens as Potential Therapeutic Agents for the Treatment of Prostate Cancer". Mini Reviews in Medicinal Chemistry. 16 (7): 531–546. doi:10.2174/1389557516666160118112448. PMID 26776222.