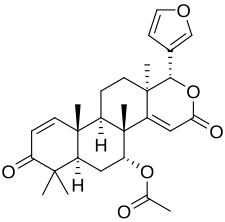
Deoxygedunin, or 14,15-deoxygedunin, is a tetranortriterpenoid isolated from the Indian neem tree[1][2] a plant that has been in traditional Indian medicine since ancient times as a remedy for various ailments.[3]
 | |
| Clinical data | |
|---|---|
| Other names | 14,15-Deoxygedunin |
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C28H34O6 |
| Molar mass | 466.574 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Pharmacology
editDeoxygedunin has been found to act as a potent, selective, small-molecule agonist of TrkB, the main receptor of brain-derived neurotrophic factor (BDNF).[1][2] It produces TrkB-dependent neurotrophic and neuroprotective effects in mice and enhances learning processes.[1][4] In addition, deoxygedunin evokes rapid TrkB-dependent antidepressant-like effects in the forced swim test, an animal model of depression, similarly to 7,8-dihydroxyflavone (7,8-DHF) and ketamine, and notably with a greater potency than 7,8-DHF.[1][2][5] The compound was discovered by the same group that identified 7,8-DHF and N-acetylserotonin as TrkB agonists.[1]
Research
editWith intraperitoneal injection to mice, deoxygedunin crosses the blood-brain-barrier into the central nervous system and possesses a long duration of action, with onset of action at 2 hours post-administration and peaking between 4–8 hours.[1] Relative to 7,8-DHF, deoxygedunin has weaker binding affinity for TrkB (Kd = 1.4 μM).[1][2] However, it is more potent than 7,8-DHF in vivo with intraperitoneal injection in multiple assays.[1] Deoxygedunin has also been found to be orally and topically active.[1][4] The compound, in contrast to 7,8-DHF, has poor water solubility,[1] and hence its bioavailability, especially oral, may be suboptimal.[6] The researchers who discovered deoxygedunin expressed that they were attempting to find analogues with improved water solubility that retained the biological activity of deoxygedunin, but, as of 2016, there appear to have been no subsequent reports on this effort since the original paper (2010) was published.[1] They also stated in the paper that 7,8-DHF has a simpler chemical structure and that the flavonoids were easier to modify for improved biological effects than the gedunins.[1]
Similarly to gedunin, a closely structurally related compound also found in Azadirachta indica, deoxygedunin has additionally been found to activate HSF1 and induce Hsp70, and was observed to possess neuroprotective effects in a model of Huntington's disease.[7]
See also
editReferences
edit- ^ a b c d e f g h i j k l Jang SW, Liu X, Chan CB, France SA, Sayeed I, Tang W, et al. (July 2010). "Deoxygedunin, a natural product with potent neurotrophic activity in mice". PLOS ONE. 5 (7): e11528. Bibcode:2010PLoSO...511528J. doi:10.1371/journal.pone.0011528. PMC 2903477. PMID 20644624.
- ^ a b c d Nordvall G, Forsell P (24 September 2014). "Stimulating neurotrophin receptors in the treatment of neurodegenerative disorders.". In Robichaud AJ (ed.). Annual Reports in Medicinal Chemistry. Elsevier Science. pp. 69–. ISBN 978-0-12-800372-5.
- ^ Vaibhav K, Shrivastava P, Khan A, Javed H, Tabassum R, Ahmed ME, et al. (August 2013). "Azadirachta indica mitigates behavioral impairments, oxidative damage, histological alterations and apoptosis in focal cerebral ischemia-reperfusion model of rats". Neurological Sciences. 34 (8): 1321–1330. doi:10.1007/s10072-012-1238-z. PMID 23187787. S2CID 2022138.
- ^ a b English AW, Liu K, Nicolini JM, Mulligan AM, Ye K (October 2013). "Small-molecule trkB agonists promote axon regeneration in cut peripheral nerves". Proceedings of the National Academy of Sciences of the United States of America. 110 (40): 16217–16222. Bibcode:2013PNAS..11016217E. doi:10.1073/pnas.1303646110. PMC 3791704. PMID 24043773.
- ^ Zhang JC, Yao W, Dong C, Yang C, Ren Q, Ma M, et al. (December 2015). "Comparison of ketamine, 7,8-dihydroxyflavone, and ANA-12 antidepressant effects in the social defeat stress model of depression". Psychopharmacology. 232 (23): 4325–4335. doi:10.1007/s00213-015-4062-3. PMID 26337614. S2CID 15076700.
- ^ Kakran M, Li L, Müller RH (July–August 2012). "Overcoming the Challenge of Poor Drug Solubility" (PDF). Pharmaceutical Engineering. Vol. 32, no. 4. International Society for Pharmaceutical Engineering.
- ^ Dinkova-Kostova AT, Zhang Y, Naidu SD, Kostov RV, Pheely A, Calabrese V (3 December 2013). "Sulfhydryl-reactive phytochemicals as dual activators of transcription factors NRF2 and HSF1.". In Gang D (ed.). 50 Years of Phytochemistry Research. Vol. 43. Springer Science & Business Media. pp. 103–. ISBN 978-3-319-00581-2.