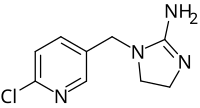
Desnitro-imidacloprid is a metabolite of the insecticide imidacloprid, a very common insecticide and the most important member of the class of insecticides called neonicotinoids, the only significant new class of insecticides to be developed between 1970 and 2000.[1] While imidacloprid has proved highly selective against insects, the desnitro- version is highly toxic to mammals, due to its agonist action at the alpha4beta2 nicotinic acetylcholine receptor (nAChR) in the mammalian brain, at least as demonstrated in experiments involving mice.[1]
| |
| Names | |
|---|---|
| IUPAC name
1-(6-Chloro-pyridin-3-yl)methyl-2-iminoimidazolidine
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C9H11ClN4 | |
| Molar mass | 210.67 g·mol−1 |
| Appearance | Colorless crystals |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
References
edit- ^ a b M. Towizawa; J.E. Casida (1 November 2002). "Desnitro-imidacloprid activates the extracellular signal-regulated kinase cascade via the nicotinic receptor and intracellular calcium mobilization in N1E-115 cells". Toxicol Appl Pharmacol. 184 (3): 180–186. doi:10.1006/taap.2002.9503. PMID 12460746.