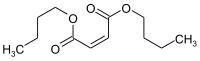
Dibutyl maleate is an organic compound with the formula (CHCO2Bu)2 (Bu = butyl). It is the diester of the unsaturated dicarboxylic acid maleic acid. It is a colorless oily liquid, although impure samples can appear yellow.
| |
| Names | |
|---|---|
| Preferred IUPAC name
Dibutyl (2Z)-but-2-enedioate | |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| 3DMet | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.003.027 |
| EC Number |
|
| MeSH | maleate dibutyl maleate |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C12H20O4 | |
| Molar mass | 228.288 g·mol−1 |
| Appearance | Colorless to yellowish liquid with a characteristic odor[1] |
| Density | 0.99 g·cm−3[1] |
| Melting point | −85 °C (−121 °F; 188 K)[1] |
| Boiling point | 280 °C (536 °F; 553 K)[1] |
| Very hardly soluble (0.17 g·l−1 at 20 °C)[1] | |
| Vapor pressure | 0.0027 hPa (20 °C)[1] |
Refractive index (nD)
|
1.445 (20 °C)[2] |
| Hazards | |
| GHS labelling: | |
   [1] [1]
| |
| H317, H373, H411[1] | |
| P273, P280, P302+P352, P314[1] | |
| Flash point | 141 °C (286 °F; 414 K)[1] |
| 265 °C (509 °F; 538 K)[1] | |
| Explosive limits | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Preparation
editDibutyl maleate can be prepared by the reaction of maleic acid anhydride and 1-butanol in presence of p-toluenesulfonic acid.[3][4]
Uses
editDibutyl maleate is mainly used as a plasticizer for aqueous dispersions of copolymers with vinyl acetate and as an intermediate in the preparation of other chemical compounds.[5] With the invention of polyaspartic technology the material found another use. In this situation, an amine is reacted with a dialkyl maleate - usually diethyl maleate but also dibutyl maleate may be used- utilizing the Michael addition reaction. The resulting products, polyaspartic esters products are then used in coatings, adhesives, sealants and elastomers.[6]
See also
editReferences
edit- ^ a b c d e f g h i j k l m n o Record of Maleinsäuredibutylester in the GESTIS Substance Database of the Institute for Occupational Safety and Health, accessed on 3 April 2019.
- ^ Sigma-Aldrich Co., Dibutyl maleate, 96%. Retrieved on 2019-04-03.
- ^ R. Wen, L. Long, L. Ding, Silas Yu (2001). "Study on synthesis of dibutyl maleate". Jishou Daxue Xuebao/Journal of Jishou University. 22 (1): 78–80.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ B. Trivedi (2013). Maleic Anhydride. Springer Science & Business Media. p. 277. ISBN 978-1-4757-0940-7.
- ^ Screening Information Dataset (SIDS) Initial Assessment Report (SIAR) for Dibutyl maletate (PDF) (Report). OECD. Retrieved 3 April 2019.
- ^ Howarth, GA (2003-06-01). "Polyurethanes, polyurethane dispersions and polyureas: Past, present and future". Surface Coatings International Part B: Coatings Transactions. 86 (2): 111–118. doi:10.1007/BF02699621. ISSN 1476-4865. S2CID 93574741.