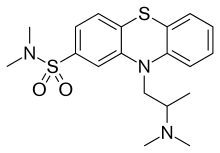
Dimetotiazine (INN) is a phenothiazine drug used for the treatment of migraine. It is a serotonin antagonist and histamine antagonist.[1]
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.028.390 |
| Chemical and physical data | |
| Formula | C19H25N3O2S2 |
| Molar mass | 391.55 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Synthesis
editThe Sandmeyer reaction on o-(4-Dimethylaminosulfonyl-2-nitrophenylthio)aniline [5510-56-5] (1) gives 4-[(2-Bromophenyl)-thio]-N,N'-dimethyl-3-nitro-benzenesulfonamide [5510-58-7] (2). The reduction of the nitro group gives 3-Amino-4-((2-bromophenyl)thio)-N,N-dimethylbenzenesulfonamide [5592-64-3] (3). Goldberg reaction gives the chief precursor, 2-Dimethylaminosulfonylphenthiazine [1090-78-4] (4). Alkylation of this with 1-chloro-N,N-dimethylpropan-2-amine [53309-35-6] (5) give Dimethothiazine (6).
References
edit- ^ Shimazawa M, Hara H, Watano T, Sukamoto T (August 1995). "Effects of Ca2+ channel blockers on cortical hypoperfusion and expression of c-Fos-like immunoreactivity after cortical spreading depression in rats". British Journal of Pharmacology. 115 (8): 1359–68. doi:10.1111/j.1476-5381.1995.tb16624.x. PMC 1908864. PMID 8564192.
- ^ GB 814512 (1959 to Rhone-Poulenc).