Fibromuscular dysplasia (FMD) is a non-atherosclerotic, non-inflammatory disease of the blood vessels that causes abnormal growth within the wall of an artery.[1] FMD has been found in nearly every arterial bed in the body, although the most commonly affected are the renal and carotid arteries.[1][2][3]
| Fibromuscular dysplasia | |
|---|---|
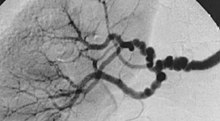 | |
| The "string-of-beads" feature in multi-focal fibromuscular dysplasia. The sign is caused by areas of relative stenoses alternating with small aneurysms. | |
| Specialty | Cardiology |
There are various types of FMD, with multi-focal fibroplasia being the most common. Less common forms of the disease include focal (previously known as intimal) and adventitial fibroplasia.[1][2][3][4] FMD predominantly affects middle-aged women, but it has been found in men and people of all ages.[1] Pediatric cases of FMD are vastly different from those of the adult population, and poorly studied. The prevalence of FMD is not known; although the disease was initially thought to be rare, some studies have suggested that it may be underdiagnosed.[5]
Signs and symptoms
editSymptoms expressed by FMD patients are largely dependent on the vascular bed(s) affected by the disease. Patients may also be entirely asymptomatic and have FMD discovered incidentally (e.g., when imaging studies are performed for other reasons). In a study from the United States Registry for Fibromuscular Dysplasia, the median age at first symptom was roughly 47 years.[2]
Renal arteries
editThe main symptoms associated with renal FMD are secondary hypertension and bruits that can be heard with a stethoscope over the abdomen or flanks. Complications such as aneurysms, dissections, or occlusion of the renal artery have been associated with renal artery FMD.[4]
Cerebrovascular regions
editThe carotid and vertebral arteries are most commonly affected. Middle and distal regions of the internal carotid arteries are frequently involved.[1] Patients with FMD in the carotid arteries typically present around 50 years of age.[3] Symptoms of craniocervical involvement include headaches (mostly migraine), pulsatile tinnitus, dizziness, and neck pain, although patients are often asymptomatic. On physical examination, one may detect neurological symptoms secondary to a stroke or transient ischemic attack (TIA), a bruit over an affected artery, and diminished distal pulses. Complications of cerebrovascular FMD include TIA, ischemic stroke, Horner syndrome, or subarachnoid hemorrhage.[1][2][3]
Other sites
editPatients with mesenteric, or intestinal, FMD may experience weight loss or abdominal pain after eating. FMD within the extremities may cause claudication or may be detectable by bruits.[1] If the lower limb arteries are affected, the patient may present with cold legs or evidence of distal embolic disease. FMD present in the subclavian artery may cause arm weakness, paresthesia, claudication, and subclavial steal syndrome.[6]
Children
editChildren with FMD often report various non-specific symptoms or present with hypertension during routine physical examinations. Symptoms are commonly associated with the artery being affected. Symptoms may include headaches, insomnia, fatigue, and chest or abdominal pain. FMD affecting the arteries of the head and neck is commonly recognized as a cause of childhood strokes.[7] In children, renovascular disease accounts for approximately 10% of all causes of secondary hypertension.[8]
Detection may stem from a bruit being present over the affected vascular bed during a physical assessment, though absence of a bruit does not preclude significant vascular disease.[8] Kidney failure is a common presentation in infants and children but is uncommon in adults, although it is occasionally the presenting problem in adults with focal disease.[3] For infants and children under four years, the presentation of FMD is "especially likely to resemble vacuities".[3]
Related diseases
editThe vascular subtype of Ehlers–Danlos Syndrome (type IV) has been associated with multi-focal FMD. This syndrome may be suspected in patients with multiple aneurysms and/or tears (dissections) in arteries, in addition to the typical angiographic findings of FMD. There have been isolated reports of FMD associated with other disorders, including Alport syndrome, pheochromocytoma, Marfan syndrome, Moyamoya disease, and Takayasu's arteritis.[9]
Cause
editWhile the cause of FMD remains unclear, current theory suggests that there may be a genetic predisposition as case reports have identified clusters of the disease and prevalence among twins.[7] According to Cleveland Clinic, approximately 10% of cases appear to be inherited and FMD often coexists with other genetic abnormalities that affect the blood vessels.[citation needed] Approximately 10% of patients with FMD have an affected family member.[1] A study conducted from the patient registry at Michigan Cardiovascular Outcomes Research and Reporting Program (MCORRP) at the University of Michigan Health System reported a high prevalence of a family history of stroke (53.5%), aneurysm (23.5%), and sudden death (19.8%).[2] Though FMD is a non-atherosclerotic disease, family histories of hypertension and hyperlipidemia were also common among those diagnosed with FMD. It is believed that there is not a single cause of FMD, but that there are multiple underlying factors. There are theories of effects of hormonal influence, mechanical stress from trauma and stress to the artery walls, and loss of oxygen supply to the blood vessel wall caused by fibrous lesions.[7] It has been suggested that environmental factors, such as smoking and estrogen, may play a role in addition to genetic factors, however concerns for safety associated with exogenous female hormones in FMD remain theoretical. [10]
Pathophysiology
editFMD can be found in almost every artery in the human body, but most often affects the carotid, vertebral, renal arteries and even those that supply the intestines, arms, and legs.[1] Patients may present with FMD in multiple vessels. FMD has been pathologically categorized into three types of classifications: multi-focal, focal, and adventitial, each referring to the particular layer of arterial wall being affected.[4]
Focal
editFocal (previously known as intimal) fibroplasia is described as long, narrow, irregular, or smooth focal stenosis and can occur in any arterial bed. Whilst it is the most common type among children, it only accounts for approximately 10% of FMD cases overall.[8] It most often presents with ischemic symptoms, and is frequently mistaken for Takayasu arteritis.[11]
Multi-focal
editMulti-focal (previously known as medial) fibroplasia involves thickening of the media and collagen formation. It is typically reported as having the appearance of a "string of beads" on angiographic review.[4] "The 'bead' component is often larger than the normal arterial lumen, and in a subset of patients with FMD, aneurysms are present that may require treatment."[4] The multi-focal subtype of FMD accounts for nearly 80% to 90% of all FMD cases.[4]
Adventitial
editIn adventitial fibroplasia, collagen replaces the fibrous adventitia and extends beyond the artery. This form is considered rare, but angiographic appearance may look similar to the focal subtype of FMD, making the distinction difficult.[citation needed]
Diagnosis
editIt is the lack of specific symptoms and their potential to appear anywhere that makes FMD a challenge to detect early on. The most accurate diagnosis comes from combining clinical presentation and angiographic imaging. According to the Michigan Cardiovascular Outcomes Research and Reporting Program (MCORRP, 2013) the length of time from a patient’s first signs or symptoms to diagnosis is commonly 5 years.[citation needed]
FMD is currently diagnosed through the use of both invasive and non-invasive tests.[10] Non-invasive testing includes duplex ultrasonography, magnetic resonance angiography (MRA), and computed tomography angiography (CTA).[4] Invasive testing through angiography is considered the best way to detect FMD, though it is typically not done early in the diagnosis process due to the higher risk of complications. Occasionally, FMD is diagnosed asymptomatically after an unrelated x-ray presents the classic "string of beads" appearance of the arteries, or when a practitioner investigates an unexpected bruit found during an exam. As part of the diagnosis process, a practitioner may review medical and family history and perform vascular examination.[citation needed]
A definitive diagnosis of FMD can only be made with imaging studies. Catheter-based angiography (with contrast) is the most accurate imaging technique; this test involves a catheter inserted into a large artery and advanced until it reaches the examined vessel.[1] The catheter allows practitioners to view and measure the pressure of the artery aiding in the categorization and severity of the FMD diseased artery. According to a study published in the Journal of Vascular Surgery, "catheter-based angiography is the only imaging modality that can accurately identify the changes of FMD, aneurysm formation, and dissection in the branch vessels."[4] Practitioners believe it is important to utilize intravascular ultrasound (IVUS) imaging because stenosis can sometimes only be detected through the methods of pressure gradient or IVUS imaging.[4] In addition, computed tomography angiography and magnetic resonance angiography are commonly used to evaluate arteries in the brain. Doppler ultrasound may be used in both the diagnosis and follow-up of FMD.[1]
Similar diseases
editIn the visceral distribution, segmental arterial mediolysis may mimic FMD. In the visceral and cerebrovascular distribution, atherosclerosis must be considered.[citation needed]
Children
editThe differentiating presentations are suggestive of FMD being a unique syndrome in respect to the pediatric population. Experienced FMD clinicians warn against relying on the "string of beads" angiography for a diagnosis.It is suggested that FMD may be both under and over-diagnosed in children with stroke.[7]
Treatment
editThere is no known cure for FMD. However, treatment focuses on relieving associated symptoms. Medical management is the most common form of treatment. The best approach to medically managing these patients is constantly being re-evaluated as more information is learned about the disease.[1]
Kidney treatment
editBlood pressure control is the primary concern when treating patients with renal FMD, as the ideal blood pressure target in patients with FMD is unknown.[10] In cases of renal artery stenosis and indications for intervention, percutaneous balloon angioplasty may be recommended. Many studies have assessed the success rate of percutaneous transluminal angioplasty (PTA) in these cases, and have found relief of hypertensive symptoms.[3][12] Duplex ultrasonography should be performed soon after this procedure to ensure adequate renal velocities.[3]
Stents have a restenosis rate of 10–20%, and may make surgical revascularization more difficult. Surgical revascularization may be necessary if aneurysms develop within the affected artery or if PTA does not resolve the issue.[citation needed]
Ex vivo renal artery reconstruction is sometimes used for complex diseases where branches of the renal artery are affected.[13]
Cerebrovascular treatment
editPatients with carotid or vertebral FMD are medically managed to reduce the risk of a stroke. Aspirin 81 mg is typically prescribed for patients with carotid FMD. Antiplatelets and anticoagulants may be used to reduce the risk of blood clot formation. If a TIA or stroke occur, percutaneous angioplasty and antiplatelet therapy may be necessary.[3] Pulsatile tinnitus is manifests in 32% of US cerebrovascular FMD patients, and sound or cognitive behavioral therapy may be helpful for some patients with more severe symptoms.[10]
Treatment for FMD in other regions
editThere is little information regarding the best treatment for FMD outside of the renal and extracranial regions. If claudication or limb ischemia is consequent to FMD in the extremities, angioplasty may be implemented.[citation needed]
Children
editIn pediatric cases, treatment is determined by factors such as age and disease location but it routinely involves controlling hypertension, re-establishing vascular flow, preventing clots, and improving lifestyle through diet, exercise, and smoking cessation. Medical therapy for pediatric population may involve the use of angiotensin-converting enzyme inhibitor (ACE inhibitors) and/or angiotensin II receptor blockers, multiple anti-hypertensive medications, diuretics, calcium channel blockers, and beta-blockers. Prevention of thrombosis of affected arteries may be taken through administration of an antiplatelet medication such as aspirin.[1]
Percutaneous transluminal renal angioplasty (PTRA) is considered the best treatment for renal-artery FMD. It is useful when hypertension is difficult to control, such as when the patient is intolerant to the anti-hypertensive medications, non-compliant to medication regime, or experiencing loss of renal volume due to ischemia. PTRA can also aid in preventing a lifelong dependency on medication. According to an article published in Cath Lab Digest, "effective PTRAs result in cured or controlled blood pressure, which is often signified by reductions in plasma renin activity and angiotensin II levels, and when compared with surgery, percutaneous balloon angioplasty is less costly, able to be performed on an outpatient basis, results in lower morbidity, and the use of stenting is not primarily necessary."[8] However, there is a subset of the pediatric population that is resistant to PTRA. Adverse events may include, "recurrent stenosis, arterial occlusion with renal loss, and arterial rupture with extravasations and pseudo aneurysm formation and may require surgical intervention."[This quote needs a citation]
Prognosis
editResearch on FMD prognoses and outcomes is scant. In some cases, if not managed properly, FMD-related aneurysms can occur and cause bleeding into the brain, resulting in a stroke, permanent nerve damage, or death. Patients with multi-focal fibroplasia generally have a favorable prognosis. Those who present with FMD in multiple vascular beds, or focal disease involving multiple branches of the renal arteries, may develop renal artery dissection [14] or progressive renal impairment, therefore having a more difficult and complex prognostic course.[3] There are no specific studies or reports on the long-term prognosis and outcome of FMD in children.[1]
References
edit- ^ a b c d e f g h i j k l m n Poloskey SL, Olin JW, Mace P, Gornik HL (May 2012). "Fibromuscular dysplasia". Circulation. 125 (18): e636–9. doi:10.1161/CIRCULATIONAHA.111.090449. PMID 22566353.
- ^ a b c d e Olin J.W.; Froehlich J.; Gu X.; et al. (2012). "The United States registry for fibromuscular dysplasia: results in the first 447 patients". Circulation. 125 (25): 3182–90. doi:10.1161/CIRCULATIONAHA.112.091223. PMID 22615343.
- ^ a b c d e f g h i j Olin JW (April 2007). "Recognizing and managing fibromuscular dysplasia". Cleveland Clinic Journal of Medicine. 74 (4): 273–4, 277–82. doi:10.3949/ccjm.74.4.273. PMID 17438676. S2CID 21928728.
- ^ a b c d e f g h i Olin JW, Sealove BA (March 2011). "Diagnosis, management, and future developments of fibromuscular dysplasia". Journal of Vascular Surgery. 53 (3): 826–36.e1. doi:10.1016/j.jvs.2010.10.066. PMID 21236620.
- ^ Hendricks NJ, Matsumoto AH, Angle JF, Baheti A, Sabri SS, Park AW, Stone JR, Patrie JT, Dworkin L, Cooper CJ, Murphy TP, Cutlip DE (October 2014). "Is fibromuscular dysplasia underdiagnosed? A comparison of the prevalence of FMD seen in CORAL trial participants versus a single institution population of renal donor candidates". Vasc Med. 19 (5): 363–7. doi:10.1177/1358863X14544715. PMID 25082538.
- ^ Lüscher TF, Lie JT, Stanson AW, Houser OW, Hollier LH, Sheps SG (October 1987). "Arterial fibromuscular dysplasia". Mayo Clin. Proc. 62 (10): 931–52. doi:10.1016/s0025-6196(12)65051-4. PMID 3309488.
- ^ a b c d Kirton A.; Crone M.; Benseler S.; Mineyko A.; Armstrong D.; Wade A.; Crous-Tsanaclis A. M. (2013). "Fibromuscular dysplasia and childhood stroke". Brain. 136 (6): 1846–56. doi:10.1093/brain/awt111. PMID 23715093.
- ^ a b c d Meyers, K.E.; Sharma, N. (2007). "Fibromuscular dysplasia in children and adolescents". Cath Lab Digest. 15 (10): 6–11.
- ^ Mace, P., "FMDSA Fibromuscular Dysplasia Society of America." Welcome to FMDSA. N.p., 2011. Web. 22 July 2013.
- ^ a b c d Gornik HL, Persu A, Adlam D, Aparicio LS, Azizi M, Boulanger M, et al. (April 2019). "First International Consensus on the diagnosis and management of fibromuscular dysplasia". Vasc Med. 24 (2): 164–189. doi:10.1177/1358863X18821816. PMID 30648921.
- ^ Tullus K (2013). "Renovascular hypertension—is it fibromuscular dysplasia or Takayasu arteritis". Pediatric Nephrology. 28 (2): 191–6. doi:10.1007/s00467-012-2151-7. PMID 22453736. S2CID 737167.
- ^ Sos TA, Pickering TG, Sniderman K, Saddekni S, Case DB, Silane MF, Vaughan ED, Laragh JH (August 1983). "Percutaneous transluminal renal angioplasty in renovascular hypertension due to atheroma or fibromuscular dysplasia". The New England Journal of Medicine. 309 (5): 274–9. doi:10.1056/NEJM198308043090504. PMID 6223227.
- ^ Belzer FO, Salvatierra O, Palubinskas A, Stoney RJ (October 1975). "Ex vivo renal artery reconstruction". Annals of Surgery. 182 (4): 456–63. doi:10.1097/00000658-197510000-00011. PMC 1344011. PMID 1180583.
- ^ Stawicki SP, Rosenfeld JC, Weger N, Fields EL, Balshi JD (September 2006). "Spontaneous renal artery dissection: three cases and clinical algorithms". J Hum Hypertens. 20 (9): 710–8. doi:10.1038/sj.jhh.1002045. PMID 16710291.
Citations
edit- Baert AL, Wilms G, Amery A, Vermylen J, Suy R (1990). "Percutaneous transluminal renal angioplasty: initial results and long-term follow-up in 202 patients". Cardiovasc Intervent Radiol. 13 (1): 22–8. doi:10.1007/BF02576933. PMID 2140294.
- Tegtmeyer CJ, Selby JB, Hartwell GD, Ayers C, Tegtmeyer V (February 1991). "Results and complications of angioplasty in fibromuscular disease". Circulation. 83 (2 Suppl): I155–61. PMID 1825043.
- Mettinger KL, Ericson K (1982). "Fibromuscular dysplasia and the brain. I. Observations on angiographic, clinical and genetic characteristics". Stroke. 13 (1): 46–52. doi:10.1161/01.str.13.1.46. PMID 7064180.
- Schievink WI (March 2001). "Spontaneous dissection of the carotid and vertebral arteries". N Engl J Med. 344 (12): 898–906. doi:10.1056/NEJM200103223441206. PMID 11259724.
- Georges A, Bouatia-Naji N (August 2022). "The complex genetic basis of fibromuscular dysplasia, a systemic arteriopathy associated with multiple forms of cardiovascular disease". Clin Sci (Lond). 136 (16): 1241–1255. doi:10.1042/CS20210990. PMC 9434409. PMID 36043395.
- Persu A, Dobrowolski P, Gornik HL, Olin JW, Adlam D, Azizi M, Boutouyrie P, Bruno RM, Boulanger M, Demoulin JB, Ganesh SK, J Guzik T, Januszewicz M, Kovacic JC, Kruk M, de Leeuw P, Loeys BL, Pappaccogli M, Perik MH, Touzé E, Van der Niepen P, Van Twist DJ, Warchoł-Celińska E, Prejbisz A, Januszewicz A (January 2022). "Current progress in clinical, molecular, and genetic aspects of adult fibromuscular dysplasia". Cardiovasc Res. 118 (1): 65–83. doi:10.1093/cvr/cvab086. PMC 8752362. PMID 33739371.
- Di Monaco S, Georges A, Lengelé JP, Vikkula M, Persu A (May 2018). "Genomics of Fibromuscular Dysplasia". Int J Mol Sci. 19 (5). doi:10.3390/ijms19051526. PMC 5983654. PMID 29883369.