Flestolol is a short-acting beta adrenergic receptor antagonist.[1]
| |
| Names | |
|---|---|
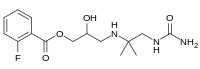
| IUPAC name
3-{[1-(Carbamoylamino)-2-methyl-2-propanyl]amino}-2-hydroxypropyl 2-fluorobenzoate
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C15H22FN3O4 | |
| Molar mass | 327.356 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Synthesis
editAcylation of acid chloride 2-Fluorobenzoyl chloride [393-52-2] (1) with glycidol (2) produces the ester 2,3-Epoxypropyl 2-Fluorobenzoate [85515-51-1] (3). Reaction of that intermediate with amine (2-Amino-2-methyl-propyl)-urea [87484-83-1] (4) obtained by reaction of 1,1-dimethylethylenediamine with urea, gives flestolol (5).
References
edit- ^ Quon, CY; Stampfli, HF (1993). "Biochemical characterization of flestolol esterase". Research communications in chemical pathology and pharmacology. 81 (3): 309–22. PMID 8235065.
- ^ Day, BW; Pento, JT; ACC-9089. Drugs Fut 1985, 10, 6, 447.
- ^ Kam, Sheung Tsam; Matier, William L.; Mai, Khuong X.; Barcelon-Yang, Cynthia; Borgman, Robert J.; O'Donnell, John P.; Stampfli, Herman F.; Sum, Check Y.; Anderson, William G. (1984). "[(Arylcarbonyl)oxy]propanolamines. 1. Novel .beta.-blockers with ultrashort duration of action". Journal of Medicinal Chemistry. 27 (8): 1007. doi:10.1021/jm00374a013. PMID 6146718.
- ^ Agustin Escobar, Dietmar M. Wagenknecht, Abu S. Alam, WO1985004581 (1985 to American Hospital Supply Corporation).