Flumethrin is a pyrethroid insecticide.[1] It is used externally in veterinary medicine against parasitic insects and ticks on cattle, sheep, goats, horses, and dogs,[2] and the treatment of parasitic mites in honeybee colonies.
| |
| Names | |
|---|---|
| IUPAC name
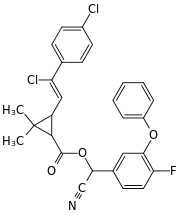
Cyano(4-fluoro-3-phenoxyphenyl)methyl 3-[2-chloro-2-(4-chlorophenyl)vinyl]-2,2-dimethylcyclopropanecarboxylate
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.067.352 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C28H22Cl2FNO3 | |
| Molar mass | 510.39 g·mol−1 |
| Pharmacology | |
| QP53AC05 (WHO) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Chemistry
editFlumethrin is a complex mixture of stereoisomers. The molecule contains three asymmetric carbon atoms, there is cis-trans isomerism at the cyclopropane ring, and cis-trans isomerism at the carbon-carbon double bond of the alkene. So there are 16 different isomers. Commercial flumethrin typically contains 92% of the trans isomers on the cyclopropane ring and the cis-configuration at the olefinic carbon-carbon double bond and 8% of the isomer with cis geometry on the cyclopropane ring and the cis-configuration at the olefinic carbon-carbon double bond.[3]
Uses
editFlumethrin is used in products, such as flea and tick collars, to protect pets against fleas.[4]
It is also used in the proprietary product, ″Bayvarol″, ″Polyvar Yellow″ which are veterinary treatments used by beekeepers against the parasitic mite Varroa destructor.
See also
editReferences
edit- ^ FLUMETHRIN, ncats.io
- ^ "4.15 Flumethrin (195) (T,R)". FAO Plant Production and Protection Papers. Food and Agriculture Organization of the United Nations. 1997.
- ^ H. J. Schnitzerling, J. Nolan und S. Hughes (1989). "Toxicology and Metabolism of Isomers of Flumethrin in Larvae of Pyrethroid-Susceptilble and Resistant Strains of the Cattle Tick Boophilus microplus (Acari: Ixodidae)". Experimental & Applied Acarology. 6 (1): 47–54. doi:10.1007/BF01193232. PMID 2707107. S2CID 1787854.
- ^ "How Soresto Works". petparents.com.