Glesatinib (MGCD265) is an experimental anti-cancer drug.[1][2]
 | |
| Clinical data | |
|---|---|
| Routes of administration | By mouth |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
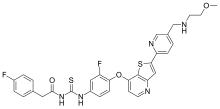
| Formula | C31H27F2N5O3S2 |
| Molar mass | 619.71 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
It is in phase 2 clinical trials for non-small cell lung cancer (NSCLC).[3]
It is a spectrum selective tyrosine[1] kinase inhibitor "for the treatment of non-small cell lung cancer (NSCLC) patients with genetic alterations of MET".[4]
See also
editReferences
edit- ^ a b "Glesatinib". NCI Dictionaries. National Cancer Institute at the National Institutes of Health. U.S. Department of Health and Human Services. Archived from the original on 2017-01-16. Retrieved 2017-01-15.
- ^ Cui Q, Cai CY, Gao HL, Ren L, Ji N, Gupta P, Yang Y, Shukla S, Ambudkar SV, Yang DH, Chen ZS (2019). "Glesatinib, a c-MET/SMO Dual Inhibitor, Antagonizes P-glycoprotein Mediated Multidrug Resistance in Cancer Cells". Frontiers in Oncology. 9: 313. doi:10.3389/fonc.2019.00313. PMC 6494935. PMID 31106148.
- ^ Clinical trial number NCT02954991 for "Phase 2 Study of Glesatinib, Sitravatinib or Mocetinostat in Combination With Nivolumab in Non-Small Cell Lung Cancer " at ClinicalTrials.gov
- ^ "Mirati Therapeutics Provides Update On Glesatinib And Sitravatinib Clinical Trials And Pipeline Programs". PipelineReview.com. January 2017.
External links
edit- glesatinib@cancer.gov Archived 2017-01-16 at the Wayback Machine