Hafnium(III) iodide is an inorganic compound of hafnium and iodine with the formula Hf I3. It is a black solid.[2]
| |
| Names | |
|---|---|
| IUPAC name
Hafnium triiodide
| |
| Identifiers | |
3D model (JSmol)
|
|
PubChem CID
|
|
| |
| |
| Properties | |
| HfI3 | |
| Molar mass | 559.20 g·mol−1 |
| Appearance | black crystals[1] |
| Melting point | decomposes |
| Related compounds | |
Other anions
|
Hafnium(III) chloride Hafnium(III) bromide |
Other cations
|
Titanium(III) iodide Zirconium(III) iodide |
Related compounds
|
Hafnium(IV) iodide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Preparation
editLike other group 4 trihalides, hafnium(III) iodide can be prepared from hafnium(IV) iodide by high-temperature reduction with hafnium metal, although incomplete reaction and contamination of the product with excess metal often occurs.[2]
- 3 Hf I4 + Hf → 4 Hf I3
Other metals can be used as the reducing agent, for example aluminium. The product is often nonstoichiometric, with the compositions Hf I3.2–3.3 and Hf I3.0–3.5 reported.[3][4]
Structure and bonding
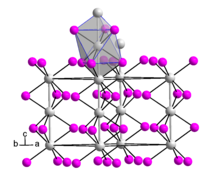
editHafnium(III) iodide adopts the same crystal structure as zirconium(III) iodide.[5] This is very similar to the β-TiCl3 structure.[2] The structure is based on hexagonal close packing of iodide ions with one third of the octahedral interstices occupied by Hf3+ ions.[2] It consists of parallel chains of face-sharing {HfI6} octahedra.[5]
Hafnium(III) iodide has a lower magnetic moment than is expected for the d1 metal ion Hf3+, indicating non-negligible Hf–Hf bonding.[2] The Hf–Hf separation was originally reported to be 3.295 Å,[6] but a subsequent study of nonstoichiometric hafnium(III) iodide indicated a lower symmetry structure.[3]
Reactivity
editLike the chloride and bromide, hafnium(III) iodide is a powerful enough reducing agent to reduce water and therefore does not have any aqueous chemistry.[2]
References
edit- ^ William M. Haynes, ed. (2013). CRC Handbook of Chemistry and Physics (93rd ed.). CRC Press. p. 4–66. ISBN 978-1466571143.
- ^ a b c d e f Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. p. 965. ISBN 978-0-08-037941-8.
- ^ a b Struss, Arthur W.; Corbett, John D. (1969). "Lower halides of hafnium. Nonstoichiometric hafnium triiodide phase". Inorg. Chem. 8 (2): 227–232. doi:10.1021/ic50072a009.
- ^ Clark, R. J. H.; Bradley, D. C.; Thornton, P. (2013). The Chemistry of Titanium, Zirconium and Hafnium Pergamon Texts in Inorganic Chemistry. Elsevier. p. 432. ISBN 978-1-4831-5921-8.
- ^ a b Wells, A. F. (1984). Structural Inorganic Chemistry (5th ed.). Oxford University Press. pp. 418–419. ISBN 978-0-19-965763-6.
- ^ Dahl, Lawrence F.; Chiang, Tao-I; Seabaugh, Pyrtle W.; Larsen, Edwin M. (1964). "Structural Studies of Zirconium Trihalides and Hafnium Triiodide". Inorg. Chem. 3 (9): 1236–1242. doi:10.1021/ic50019a008.