| |
| Names | |
|---|---|
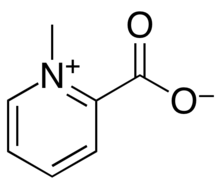
| Preferred IUPAC name
1-Methylpyridin-1-ium-2-carboxylate | |
| Other names
N-methyl picolinic acid betaine, Betaine homarine
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C7H7NO2 | |
| Molar mass | 137.138 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Homarine (N-methyl picolinic acid betaine) is an organic compound with the chemical formula C7H7NO2.[2] It is commonly found in aquatic organisms from phytoplankton to crustaceans, although it is not found in vertebrates.[3][4]
Biological function
editHomarine functions as an osmolyte by affecting the ionic strength of the cytosol and thereby maintaining osmotic pressure within the cell.[5]
Homarine may also act as a methyl group donor in the biosynthesis of various other N-methylated chemicals, such as glycine betaine and choline. The process of methyl donation converts homarine into picolinic acid and is reversible.[6]
Etymology
editThe name of this chemical comes from the initial discovery of the molecule in 1933 in lobster tissue:[4] the word homarine as an adjective means "of, or relating to, lobsters" (i.e. genus Homarus).
References
edit- ^ "Homarine". pubchem.ncbi.nlm.nih.gov. Retrieved 1 October 2020.
- ^ Hoppe-Seyler, F. A. (January 1933). "Über das Homarin, eine bisher unbekannte tierische Base". Hoppe-Seyler's Zeitschrift für physiologische Chemie. 222 (3–4): 105–115. doi:10.1515/bchm2.1933.222.3-4.105.
- ^ Dickson, D. M. J.; Kirst, G. O. (August 1987). "Osmotic Adjustment in Marine Eukaryotic Algae: The Role of Inorganic Ions, Quaternary Ammonium, Tertiary Sulphonium and Carbohydrate Solutes. I. Diatoms and a Rhodophyte". New Phytologist. 106 (4): 645–655. doi:10.1111/j.1469-8137.1987.tb00165.x.
- ^ a b Gasteiger, E. L.; Haake, P. C.; Gergen, J. A. (15 December 2006). "An Investigation of the Distribution and Function of Homarine (N-Methyl Picolinic Acid)". Annals of the New York Academy of Sciences. 90 (3): 622–636. doi:10.1111/j.1749-6632.1960.tb26410.x. PMID 13703887.
- ^ Gebser, Björn; Pohnert, Georg (17 June 2013). "Synchronized Regulation of Different Zwitterionic Metabolites in the Osmoadaption of Phytoplankton". Marine Drugs. 11 (6): 2168–2182. doi:10.3390/md11062168. PMC 3721227. PMID 23774888.
- ^ Netherton, James; Gurin, Samuel (1982). "Biosynthesis and Physiological Role of Homarine in Marine Shrimp". Journal of Biological Chemistry. 257 (20): 11971–11975. PMID 7118923. Retrieved 1 October 2020.