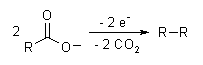The Kolbe electrolysis or Kolbe reaction is an organic reaction named after Hermann Kolbe.[1] The Kolbe reaction is formally a decarboxylative dimerisation of two carboxylic acids (or carboxylate ions). The overall reaction is:
If a mixture of two different carboxylates are used, all combinations of them are generally seen as the organic product structures:
- 3 R1COO− + 3 R2COO− → R1−R1 + R1−R2 + R2−R2 + 6 CO2 + 6 e−
The reaction mechanism involves a two-stage radical process: electrochemical decarboxylation gives a radical intermediate, which combine to form a covalent bond.[2] As an example, electrolysis of acetic acid yields ethane and carbon dioxide:
- CH3COOH → CH3COO− → CH3COO· → CH3· + CO2
- 2CH3· → CH3CH3
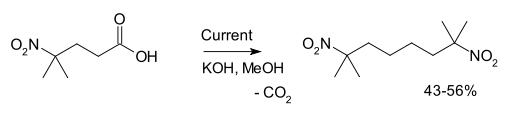
Another example is the synthesis of 2,7-dimethyl-2,7-dinitrooctane from 4-methyl-4-nitrovaleric acid:[3]
The Kolbe reaction has also been occasionally used in cross-coupling reactions.
In 2022, it was discovered that the Kolbe electrolysis is enhanced if an alternating square wave current is used instead of a direct current.[4][5]
Applications
editKolbe electrolysis has a few industrial applications.[6] In one example, sebacic acid has been produced commercially by Kolbe electrolysis of adipic acid.[7]
Kolbe electrolysis has been examined for converting biomass into biodiesel[8][9] and for grafting of carbon electrodes.[10][11]
See also
editReferences
edit- ^ Utley, James (1997). "Trends in Organic Electrosynthesis". Chemical Society Reviews. 26 (3): 157. doi:10.1039/cs9972600157.
- ^ Vijh, A. K.; Conway, B. E. (1967). "Electrode Kinetic Aspects of the Kolbe Reaction". Chem Rev. 67 (6): 623–664. doi:10.1021/cr60250a003.
- ^ Sharkey, W. H.; Langkammerer, C. M. (1973). "2,7-Dimethyl-2,7-dinitrooctane". Organic Syntheses; Collected Volumes, vol. 5, p. 445.
- ^ Hioki, Yuta; Costantini, Matteo; Griffin, Jeremy; Harper, Kaid; Prado Merini, Melania; Nissl, Benedikt; Kawamata, Yu; Baran, Phil (31 October 2022). Overcoming the Limitations of Kolbe Coupling via Waveform-Controlled Electrosynthesis (Report). Chemistry. doi:10.26434/chemrxiv-2022-3cj82-v2.
- ^ Hioki, Yuta; Costantini, Matteo; Griffin, Jeremy; Harper, Kaid C.; Merini, Melania Prado; Nissl, Benedikt; Kawamata, Yu; Baran, Phil S. (7 April 2023). "Overcoming the limitations of Kolbe coupling with waveform-controlled electrosynthesis". Science. 380 (6640): 81–87. doi:10.1126/science.adf4762. ISSN 0036-8075. PMID 37023204.
- ^ Wendt, Hartmut; Vogt, Helmut; Kreysa, Gerhard; m. Kolb, Dieter; e. Engelmann, Gerald; Ziegler, Jörg C.; Goldacker, Hubert; Jüttner, Klaus; Galla, Ulrich; Schmieder, Helmut; Steckhan, Eberhard (2009). "Electrochemistry". Ullmann's Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.a09_183.pub3. ISBN 978-3-527-30673-2.
- ^ Seko, Maomi; Yomiyama, Akira; Isoya, Toshiro (1979). "Development of Kolbe Electrosynthesis of Sebacic Acid". CEER, Chemical Economy & Engineering Review. 11 (9): 48–50.
- ^ Yuan, Gang; Wu, Chan; Zeng, Guorong; Niu, Xiaopo; Shen, Guoqiang; Wang, Li; Zhang, Xiangwen; Luque, Rafael; Wang, Qingfa (18 January 2020). "Kolbe Electrolysis of Biomass-Derived Fatty Acids Over Pt Nanocrystals in an Electrochemical Cell". ChemCatChem. 12 (2): 642–648. doi:10.1002/cctc.201901443. ISSN 1867-3880.
- ^ Ho, Calvin K.; McAuley, Kimberley B.; Peppley, Brant A. (1 October 2019). "Biolubricants through renewable hydrocarbons: A perspective for new opportunities". Renewable and Sustainable Energy Reviews. 113: 109261. doi:10.1016/j.rser.2019.109261. ISSN 1364-0321.
- ^ Andrieux, Claude P.; Gonzalez, Felipe; Savéant, Jean-Michel (1 May 1997). "Derivatization of Carbon Surfaces by Anodic Oxidation of Arylacetates. Electrochemical Manipulation of the Grafted Films". Journal of the American Chemical Society. 119 (18): 4292–4300. doi:10.1021/ja9636092. ISSN 0002-7863.
- ^ Bélanger, Daniel; Pinson, Jean (20 June 2011). "Electrografting: a powerful method for surface modification". Chemical Society Reviews. 40 (7): 3995–4048. doi:10.1039/C0CS00149J. ISSN 1460-4744. PMID 21503288.
Further reading
edit- Kolbe, Hermann (1848). "Zersetzung der Valeriansäure durch den elektrischen Strom" [Decomposition of valeric acid by an electric current]. Annalen der Chemie und Pharmacie. 64 (3): 339–341. doi:10.1002/jlac.18480640346.
- Kolbe, Hermann (1849). "Untersuchungen über die Elektrolyse organischer Verbindungen" [Investigations of the electrolysis of organic compounds]. Annalen der Chemie und Pharmacie. 69 (3): 257–294. doi:10.1002/jlac.18490690302.
External links
edit- "Kolbe Electrolysis". Organic Chemistry Portal. Retrieved 22 October 2007.