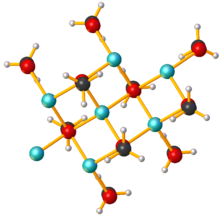
Lithium methoxide is a compound with formula LiCH3O. It is the lithium salt of methanol. Like other alkali metal alkoxides, lithium methoxide adopts a polymeric structure[1] Its solubility in common polar aprotic solvents like THF is low; however, it is soluble in methanol and is available commercially as a 10% solution.[2]
 | |
| Names | |
|---|---|
| IUPAC name
Lithium methoxide
| |
| Other names
Lithium methanolate
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.011.580 |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| CH3LiO | |
| Molar mass | 37.975 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
See also
editReferences
edit- ^ WHEATLEY, P. J. (1960-03-05). "Structure of Lithium Methoxide". Nature. 185 (4714): 681–682. Bibcode:1960Natur.185..681W. doi:10.1038/185681b0. S2CID 6915735.
- ^ "Lithium Methoxide (LiOMe) in 10% Methanol" (PDF). FMC lithium. 2016. Archived from the original (PDF) on 6 September 2017. Retrieved 6 Sep 2017.