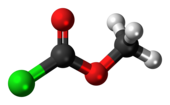
Methyl chloroformate is a chemical compound with the chemical formula Cl−C(=O)−O−CH3. It is the methyl ester of chloroformic acid. It is an oily colorless liquid, although aged samples appear yellow. It is also known for its pungent odor.

| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
Methyl carbonochloridate | |
| Other names
Methyl chloroformate, Chlorocarbonic methyl ester, Methyl chlorocarbonate
| |
| Identifiers | |
3D model (JSmol)
|
|
| 605437 | |
| ChemSpider | |
| ECHA InfoCard | 100.001.080 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| ClC(O)OCH3 | |
| Molar mass | 94.49 g·mol−1 |
| Appearance | Colorless oily liquid |
| Odor | Pungent |
| Density | 1.223 g/mL |
| Boiling point | 70 to 72 °C (158 to 162 °F; 343 to 345 K) |
| Hazards | |
| GHS labelling: | |
  
| |
| Danger | |
| H225, H302, H312, H314, H330 | |
| P210, P233, P240, P241, P242, P243, P260, P264, P270, P271, P280, P284, P301+P312, P301+P330+P331, P302+P352, P303+P361+P353, P304+P340, P305+P351+P338, P310, P312, P320, P321, P322, P330, P363, P370+P378, P403+P233, P403+P235, P405, P501 | |
| Flash point | 10 °C (50 °F; 283 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Preparation
editMethyl chloroformate can be synthesized using anhydrous methanol and phosgene.[2]
- COCl2 + CH3OH → ClC(O)OCH3 + HCl
Properties
editMethyl chloroformate hydrolyzes in water to form methanol, hydrochloric acid, and carbon dioxide.[3] This decomposition happens violently in the presence of steam, causing foaming. The compound decomposes in heat, which can liberate hydrogen chloride, phosgene, chlorine, or other toxic gases.[4]
Uses
editMethyl chloroformate is used in organic synthesis for the introduction of the methoxycarbonyl functionality to a suitable nucleophile (i.e. carbomethoxylation).[5]
Safety
editMethyl chloroformate forms highly flammable vapour-air mixtures. The compound has a flash point of 10 °C.[6] Methyl chloroformate, if heated, releases phosgene. It produces hydrogen chloride upon contact with water. It will cause skin damage if in contact with skin.
See also
editReferences
edit- ^ Methyl chloroformate at Sigma-Aldrich
- ^ Siegfried Böhm, Maren Beth-Hübner: Chloroformic Esters. In: Ullmann’s Encyclopedia of Industrial Chemistry. Wiley‐VCH Verlag GmbH & Co. KGaA., 15. April 2006, doi:10.1002/14356007.a06_559.pub2.
- ^ "Toxokologische Bewertungen" [Toxicological Information] (PDF) (in German). Retrieved 2021-10-03.
- ^ "Methylchlorformiat". GESTIS-Stoffdatenbank (in German). Retrieved 2021-10-03.
- ^ Fischer, Emil (1914). "Synthesis of depsides, lichen-substances and tannins". Journal of the American Chemical Society. 36 (6): 1170–1201. doi:10.1021/ja02183a009.
- ^ E. Brandes, W. Möller: Sicherheitstechnische Kenngrößen. Band 1: Brennbare Flüssigkeiten und Gase. Wirtschaftsverlag NW – Verlag für neue Wissenschaft, Bremerhaven 2003.