| Structural formula | 
|

|

|
|
| Name | Fluoromethane Methyl fluoride |
Chloromethane Methyl chloride |
Bromomethane Methyl bromide |
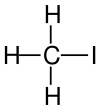
Iodomethane Methyl iodide |
| Melting point | −137,8 °C[1] | −97,4 °C[2] | −93,7 °C[3] | −66 °C[4] |
| Boiling point | −78,4 °C[1] | −23,8 °C[2] | 4,0 °C[3] | 42 °C[4] |
| Space-filling model | 
|

|

|

|
The monohalomethanes are organic compounds in which a hydrogen atom in methane is replaced by a halogen. They belong to the haloalkanes or to the subgroup of halomethanes.
The four common[a] members are fluoromethane, chloromethane, bromomethane and iodomethane.
Historical name for this group is methyl halides; it's still widely used. The compounds of this class are often described as CH3X or MeX (X - any halogen, Me - methyl group).
Related compounds
editThere are analogs with more than one hydrogen atom in methane is replaced by a halogen:
- Dihalomethane, CH2X2, two hydrogen atoms replaced
- Trihalomethane, CHX3, three hydrogen atoms replaced
- Tetrahalomethane, CX4, all four hydrogen atoms replaced
Analogs with carbon atom replaced with a heavier group 14 element are also known:
See also
edit- Methyl halide transferase, an enzyme producing some methyl halides
Notes
edit- ^ Highly radioactive CH3At (methyl astatide) has been detected.[5] The known isotopes of even heavier group 17 element, tennessine, are too short-lived to allow for chemical experimentation.
References
edit- ^ a b Record of Fluoromethane in the GESTIS Substance Database of the Institute for Occupational Safety and Health, accessed on 2020-02-29.
- ^ a b Record of Chloromethane in the GESTIS Substance Database of the Institute for Occupational Safety and Health, accessed on 2020-02-29.
- ^ a b Record of Bromomethane in the GESTIS Substance Database of the Institute for Occupational Safety and Health, accessed on 2020-02-29.
- ^ a b Record of Iodomethane in the GESTIS Substance Database of the Institute for Occupational Safety and Health, accessed on 2020-02-29.
- ^ "Chemical Fact Sheet: Astatine". European Virtual Institute for Speciation Analysis (EVISA).
Wikimedia Commons has media related to Monohalomethanes.