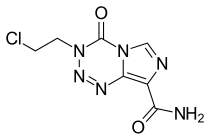
Mitozolomide (INN) is an antineoplastic. It is an imidazotetrazine derivative.
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.079.921 |
| Chemical and physical data | |
| Formula | C7H7ClN6O2 |
| Molar mass | 242.62 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Development of mitozolomide was discontinued during Phase II clinical trials after it was found to cause severe and unpredictable bone marrow suppression.[1] Temozolomide, which has been in clinical use since 1999, is a less toxic analogue of mitozolomide.[2]
References
edit- ^ Fairbairn LJ, Chinnasamy N, Lashford LS, Chinnasamy D, Rafferty JA (February 2000). "Enhancing hemopoietic drug resistance: a rationale for reconsidering the clinical use of mitozolomide" (PDF). Cancer Gene Ther. 7 (2): 233–9. doi:10.1038/sj.cgt.7700120. PMID 10770631. S2CID 2597751.
- ^ Newlands ES, Blackledge GR, Slack JA, et al. (February 1992). "Phase I trial of temozolomide (CCRG 81045: M&B 39831: NSC 362856)". Br J Cancer. 65 (2): 287–91. doi:10.1038/bjc.1992.57. PMC 1977719. PMID 1739631.