An NIH shift is a chemical rearrangement where a hydrogen atom on an aromatic ring undergoes an intramolecular migration primarily during a hydroxylation reaction. This process is also known as a 1,2-hydride shift. These shifts are often studied and observed by isotopic labeling. An example of an NIH shift is shown below:
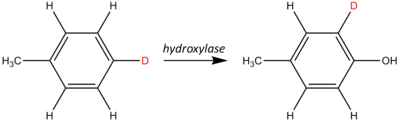
In this example, a hydrogen atom has been isotopically labeled using deuterium (shown in red). As the hydroxylase adds a hydroxyl (the −OH group), the labeled site shifts one position around the aromatic ring relative to the stationary methyl group (−CH3).
Several hydroxylase enzymes are believed to incorporate an NIH shift in their mechanism, including 4-hydroxyphenylpyruvate dioxygenase and the tetrahydrobiopterin dependent hydroxylases. The name NIH shift arises from the US National Institutes of Health from where studies first reported observing this transformation.
References
edit- Guroff, G.; Daly, J.W.; Jerina, D.M.; Renson, J.; Witkop, B.; Udenfriend, S. (1967). "Hydroxylation-induced migration: the NIH shift. Recent experiments reveal an unexpected and general result of enzymatic hydroxylation of aromatic compounds". Science. 157 (3796): 1524–1530. Bibcode:1967Sci...157.1524G. doi:10.1126/science.157.3796.1524. PMID 6038165..
- Bassan, A.; Blomberg, M.R.A.; Siegbahn, P.E.M. (2003). "Mechanism of Aromatic Hydroxylation by an Activated FeIV=O Core in Tetrahydrobiopterin-Dependent Hydroxylases". Chem. Eur. J. 9 (17): 4055–4067. doi:10.1002/chem.200304768. PMID 12953191..