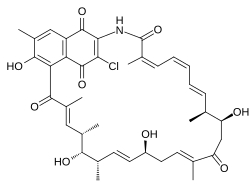
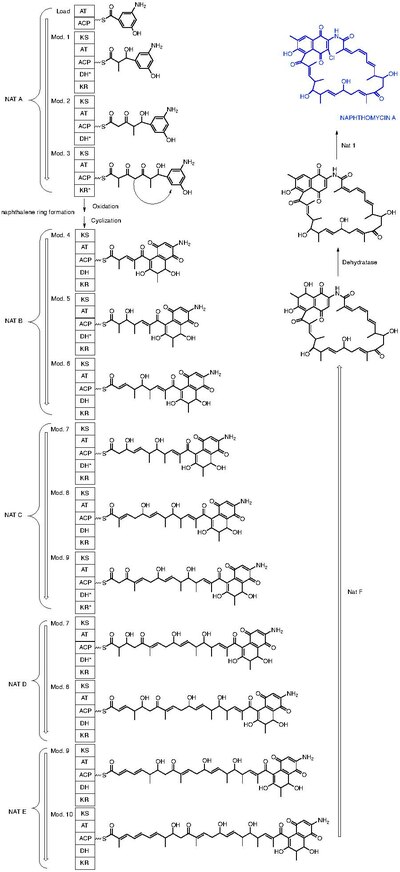
Naphthomycin A is a type of naphthomycin. It was isolated as a yellow pigment from Streptomyces collinus and it shows antibacterial, antifungal, and antitumor activities.[1] Naphthomycins have the longest ansa aliphatic carbon chain of the ansamycin family.[2] Biosynthetic origins of the carbon skeleton from PKS1 were investigated by feeding 13C-labeled precursors and subsequent 13C-NMR product analysis. Naphthomycin gene clusters have been cloned and sequenced to confirm involvement in biosynthesis via deletion of a 7.2kb region.[3] Thirty-two genes were identified in the 106kb cluster.[4]
| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| C40H46ClNO9 | |
| Molar mass | 720.26 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
References
edit- ^ Kang, Q.; Y. Shenb; L. Bai (2012). "Biosynthesis of 3,5-AHBA-derived natural products". Nat. Prod. Rep. 29 (2): 243–263. doi:10.1039/c2np00019a. PMID 22193711.
- ^ Balerna, M.; W. Keller-Schierlein; C. Martius; H. Zahner (1969). "Stoffwechselprodukte von Mikroorganismen". Arch. Microbiol. 65 (4): 303–317. doi:10.1007/BF00412210. PMID 4988744. S2CID 31145406.
- ^ Bai, L.; Y. Wu; Q. Kang; Y. Shen; Z. Deng (2012). Patent. CN: 2012-10300294: 225.
{{cite journal}}: Missing or empty|title=(help) - ^ August, P.R.; L. Tang; Y.J. Yoon; S. Ning; R. MuEller; T.W. Yu; M. Taylor; D. Hoffman; C.G. Kim; X. Zahng; C.R. Hutchinson; H.G. Floss (1998). "Biosynthesis of the ansamycin antibiotic rifamycin: Deductions from the molecular analysis of the rif biosynthetic gene cluster of Amycolatopsis mediterranei S699". Chem. Biol. 5 (2): 69–79. doi:10.1016/S1074-5521(98)90141-7. PMID 9512878.
- ^ Kang, Q; Y. Shenb; L. Bai (2012). "Biosynthesis of 3,5-AHBA-derived natural products". Nat. Prod. Rep. 29 (2): 243–263. doi:10.1039/c2np00019a. PMID 22193711.