Neoxanthin is a carotenoid and xanthophyll. In plants, it is an intermediate in the biosynthesis of the plant hormone abscisic acid. It is often present in two forms: all-trans and 9-cis isomers. It is produced from violaxanthin, but a suspected neoxanthin synthase[1] is still to be confirmed. Two different genes were confirmed to be implied in violaxanthin conversion to neoxanthin in Arabidopsis and tomato.[2][3] It has a specific role in protection against photooxidative stress.[4] It is a major xanthophyll found in green leafy vegetables such as spinach.

| |
| Names | |
|---|---|
| IUPAC name
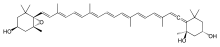
(1R,3S)-6-((R,3E,5E,7E,9E,11E,13E,15Z,17E)-18-((1S,4S,6R)-4-hydroxy-2,2,6-trimethyl-7-oxabicyclo[4.1.0]heptan-1-yl)-3,7,12,16-tetramethyloctadeca-1,3,5,7,9,11,13,15,17-nonaen-1-ylidene)-1,5,5-trimethylcyclohexane-1,3-diol
| |
| Other names
Foliaxanthin; Neoxanthine
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C40H56O4 | |
| Molar mass | 600.884 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
References
edit- ^ Bouvier, Florence; D'harlingue, Alain; Backhaus, Ralph A.; Kumagai, Monto H.; Camara, Bilal (2000). "Identification of neoxanthin synthase as a carotenoid cyclase paralog". European Journal of Biochemistry. 267 (21): 6346–6352. doi:10.1046/j.1432-1327.2000.01722.x. PMID 11029576.
- ^ North, Helen M.; de Almeida, Aurélie; Boutin, Jean-Pierre; Frey, Anne; To, Alexandra; Botran, Lucy; Sotta, Bruno; Marion-Poll, Annie (2007). "The Arabidopsis ABA-deficient mutant aba4 demonstrates that the major route for stress-induced ABA accumulation is via neoxanthin isomers". Plant Journal. 50 (5): 810–824. doi:10.1111/j.1365-313X.2007.03094.x. PMID 17470058.
- ^ Neuman, Hadar; Galpaz, Navot; Cunningham Jr, Francis X.; Zamir, Dani; Hirschberg, Joseph (2014). "The tomato mutation nxd1 reveals a gene necessary for neoxanthin biosynthesis and demonstrates that violaxanthin is a sufficient precursor for abscisic acid biosynthesis". Plant Journal. 78 (1): 80–93. doi:10.1111/tpj.12451. PMID 24506237.
- ^ Dall'osto, Luca; Cazzaniga, Stefano; North, Helen; Marion-Poll, Annie; Bassi, Roberto (2007). "The Arabidopsis aba4-1 mutant reveals a specific function for neoxanthin in protection against photooxidative stress". Plant Cell. 19 (3): 1048–1064. doi:10.1105/tpc.106.049114. PMC 1867355. PMID 17351115.