Nicotryine is lesser known and minor tobacco alkaloid. It inhibits metabolism of nicotine[2][3] through CYP2A6 enzyme inhibition (Ki = 7.5 ± 2.9).[2][4][5] It also inhibits CYP2A13 (Ki = 5.6 ± 0.86) which might play role in nicotine metabolism.[5][6] Nicotyrine is formed by gradual oxidation of nicotine in e-liquids and causes delayed nicotine clearance and attenuated withdrawal symptoms.[6]
| |
| Names | |
|---|---|
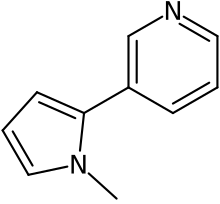
| IUPAC name
3-(1-methylpyrrol-2-yl)pyridine
| |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.006.956 |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C10H10N2 | |
| Molar mass | 158.204 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
It has insecticidal properties like nicotine and certain derivatives have been synthesized for that property.[7]
Chemistry
editalpha-nicotyrine and beta-nicotyrine are positional isomers of each other.
Synthesis
editNicotyrine can be produced readily from nicotine by catalytic dehydrogenation[8] and from tobacco biomass by catalytic pyrolysis.[9]
See also
editReferences
edit- ^ "Alpha-nicotyrine - MeSH - NCBI".
- ^ a b Denton TT, Zhang X, Cashman JR (February 2004). "Nicotine-related alkaloids and metabolites as inhibitors of human cytochrome P-450 2A6". Biochemical Pharmacology. 67 (4): 751–6. doi:10.1016/j.bcp.2003.10.022. PMID 14757175.
- ^ Stålhandske T, Slanina P (September 1982). "Nicotyrine inhibits in vivo metabolism of nicotine without increasing its toxicity". Toxicology and Applied Pharmacology. 65 (3): 366–72. doi:10.1016/0041-008x(82)90382-9. PMID 7157369.
- ^ Kramlinger VM, von Weymarn LB, Murphy SE (May 2012). "Inhibition and inactivation of cytochrome P450 2A6 and cytochrome P450 2A13 by menthofuran, β-nicotyrine and menthol". Chemico-Biological Interactions. 197 (2–3): 87–92. doi:10.1016/j.cbi.2012.03.009. PMC 3362486. PMID 22486895.
- ^ a b Stephens ES, Walsh AA, Scott EE (September 2012). "Evaluation of inhibition selectivity for human cytochrome P450 2A enzymes". Drug Metabolism and Disposition. 40 (9): 1797–802. doi:10.1124/dmd.112.045161. PMC 3422547. PMID 22696418.
- ^ a b Abramovitz A, McQueen A, Martinez RE, Williams BJ, Sumner W (September 2015). "Electronic cigarettes: The nicotyrine hypothesis". Medical Hypotheses. 85 (3): 305–10. doi:10.1016/j.mehy.2015.06.002. PMID 26100465.
- ^ Frank, Robert L.; Holley, Robert W.; Wikholm, Donald M. (2002). "3,2'-Nicotyrine. Insecticidal Properties of Certain Azo Derivatives1". Journal of the American Chemical Society. 64 (12): 2835–2838. doi:10.1021/ja01264a033. ISSN 0002-7863.
- ^ SHIBAGAKI, Makoto; TAKAHASHI, Kyoko; KUNO, Hideyuki; MATSUSHITA, Hajime (1988). "Preparation of nicotyrine via catalytic dehydrogenation of nicotine". Agricultural and Biological Chemistry. 52 (10): 2651–2652. doi:10.1271/bbb1961.52.2651. ISSN 0002-1369.
- ^ Ye, Xiao-ning; Lu, Qiang; Li, Wen-tao; Gao, Pan; Hu, Bin; Zhang, Zhi-bo; Dong, Chang-qing (2016). "Selective production of nicotyrine from catalytic fast pyrolysis of tobacco biomass with Pd/C catalyst". Journal of Analytical and Applied Pyrolysis. 117: 88–93. doi:10.1016/j.jaap.2015.12.012. ISSN 0165-2370.