Octadecaborane is an inorganic compound, a boron hydride cluster with chemical formula B18H22. It is a colorless flammable solid, like many higher boron hydrides. Although the compound has no practical applications, its structure is of theoretical and pedagogical interest.

| |
| |
| Names | |
|---|---|
| Other names
octadecaborane; octadecaboron doicosahydride; octodecaborane; n-Octadecaborane; i-Octadecaborane
| |
| Identifiers | |
| ECHA InfoCard | 100.224.871 |
| EC Number |
|
| Properties | |
| B18H22 | |
| Molar mass | 216.77 g/mol |
| Appearance | White to off white powder |
| Density | 1.012 g/cm3 |
| Melting point | 180 and 129 °C (356 and 264 °F; 453 and 402 K) n-B18H22 and i-B18H22 respectively |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Synthesis
editIt is formed by oxidative degradation of B20H182− or by oxidative coupling of B9H12−.
Structure
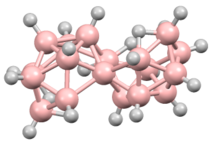
editTwo isomers are known of octadecaborane, providing the first example of isomers in a boron-hydride cluster. The clusters are also of interest because the boron centers shared between the two subunits have an unusually high number of B-B interactions. The isomers consists of two B9H11 polyhedral subunits, each having a decaborane-like form, joined at a B–B edge.[1][2] These two boron atoms are each coordinated to six others; this compound was the first one found to have such a high number of borons coordinated around a single boron center.[3] There are two different geometric isomers of this compound, differing in the orientation of the two edge-fused polyhedra to each other.[1][2] This compound was the first borane found to have multiple isomeric forms.[4] Among the geometric isomers, one with chirality was the first borane to be resolved into its separate enantiomers, and was only the second chiral borane known at that time.[5]
References
edit- ^ a b Olsen, Frederic P.; Vasavada, Ravindra C.; Hawthorne, M. Frederick (1968). "The chemistry of n-B18H22 and i-B18H22". J. Am. Chem. Soc. 90 (15): 3946–3951. doi:10.1021/ja01017a007.
- ^ a b Londesborough, Michael G.S.; Hnyk, Drahomír; Bould, Jonathan; Serrano-Andrés, Luis; Sauri, Vicenta; Oliva, Josep M.; Kubát, Pavel; Polívka, Tomáš; Lang, Kamil (2012). "Distinct Photophysics of the Isomers of B18H22 Explained". Inorg. Chem. 51 (3): 1471–1479. doi:10.1021/ic201726k. hdl:10261/92295. PMID 22224484.
- ^ Simpson, P. G.; Lipscomb, W. N. (1962). "Molecular Structure of B18H22" (PDF). Proceedings of the National Academy of Sciences of the United States of America. 48 (9): 1490–1491. Bibcode:1962PNAS...48.1490S. doi:10.1073/pnas.48.9.1490. PMC 220984. PMID 16590990.
- ^ Simpson, Paul G.; Folting, Kirsten; Lipscomb, William N. (1963). "The Molecular Structure of i-B18H22". J. Am. Chem. Soc. 85 (12): 1879–1880. doi:10.1021/ja00895a046.
- ^ Heřmánek, S.; Plešek, J. (1970). "Chemistry of boranes. XXI. Resolution of iso-octadecaborane to optical enantiomers". Collection of Czechoslovak Chemical Communications. 35 (8): 2488–2493. doi:10.1135/cccc19702488.