The enzyme ornithine cyclodeaminase (EC 4.3.1.12) catalyzes the chemical reaction
| ornithine cyclodeaminase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| EC no. | 4.3.1.12 | ||||||||
| CAS no. | 9054-76-6 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| Gene Ontology | AmiGO / QuickGO | ||||||||
| |||||||||
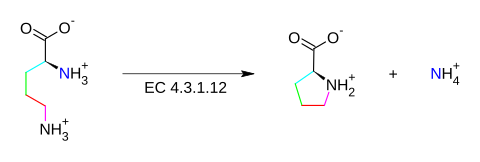
L-ornithine L-proline + NH4+
This enzyme belongs to the family of lyases, specifically ammonia lyases, which cleave carbon-nitrogen bonds. The systematic name of this enzyme class is Lornithine ammonia-lyase (cyclizing; L-proline-forming). Other names in common use include ornithine cyclase, ornithine cyclase (deaminating), and L-ornithine ammonia-lyase (cyclizing). This enzyme participates in arginine and proline biosynthesis. It employs one cofactor, NAD+.
Structural studies
editAs of late 2007, two structures have been solved for this class of enzymes, with PDB accession codes 1U7H and 1X7D.
References
edit- Costilow RN, Laycock L (1971). "Ornithine cyclase (deaminating). Purification of a protein that converts ornithine to proline and definition of the optimal assay conditions". Journal of Biological Chemistry. 246 (21): 6655–60. doi:10.1016/S0021-9258(19)34165-1. PMID 4399881.
- Muth WL, Costilow RN (1974). "Ornithine cyclase (deaminating). II. Properties of the homogeneous enzyme". Journal of Biological Chemistry. 249 (23): 7457–62. doi:10.1016/S0021-9258(19)81260-7. PMID 4373469.
- Espineda CE, Linford AS, Devine D, Brusslan JA (1999). "The AtCAO gene, encoding chlorophyll a oxygenase, is required for chlorophyll b synthesis in Arabidopsis thaliana". Proceedings of the National Academy of Sciences of the United States of America. 96 (18): 10507–11. Bibcode:1999PNAS...9610507E. doi:10.1073/pnas.96.18.10507. PMC 17919. PMID 10468639.
- Goodman JL, Wang S, Alam S, Ruzicka FJ, Frey PA, Wedekind JE (2004). "Ornithine cyclodeaminase: structure, mechanism of action, and implications for the mu-crystallin family". Biochemistry. 43 (44): 13883–91. doi:10.1021/bi048207i. PMID 15518536.
- Alam S, Wang SC, Ruzicka FJ, Frey PA, Wedekind JE (2004). "Crystallization and X-ray diffraction analysis of ornithine cyclodeaminase from Pseudomonas putida". Acta Crystallographica Section D. 60 (Pt 5): 941–4. doi:10.1107/S0907444904005256. PMID 15103146.
