Palmitoylcarnitine is an ester derivative of carnitine involved in the metabolism of fatty acids. During the tricarboxylic acid cycle (TCA), fatty acids undergo a process known as β-oxidation to produce energy in the form of ATP. β-oxidation occurs primarily within mitochondria, however the mitochondrial membrane prevents the entry of long chain fatty acids (>C10), so the conversion of fatty acids such as palmitic acid is key.[1] Palmitic acid is brought to the cell and once inside the cytoplasm is first converted to Palmitoyl-CoA. Palmitoyl-CoA has the ability to freely pass the outer mitochondrial membrane, but the inner membrane is impermeable to the Acyl-CoA and thioester forms of various long-chain fatty acids such as palmitic acid. The palmitoyl-CoA is then enzymatically transformed into palmitoylcarnitine via the Carnitine O-palmitoyltransferase family. The palmitoylcarnitine is then actively transferred into the inner membrane of the mitochondria via the carnitine-acylcarnitine translocase.[2] Once inside the inner mitochondrial membrane, the same Carnitine O-palmitoyltransferase family is then responsible for transforming the palmitoylcarnitine back to the palmitoyl-CoA form.
| |
| Names | |
|---|---|
| IUPAC name
3-(palmitoyloxy)-4-(trimethylammonio)butanoate
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| MeSH | Palmitoylcarnitine |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C23H45NO4 | |
| Molar mass | 399.608 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Structure
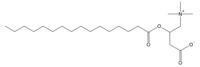
editPalmitoylcarnitine contains the saturated fatty acid known as palmitic acid (C16:0) which is bound to the β-hydroxy group of the carnitine. The core carnitine structure, consisting of butanoate with a quaternary ammonium attached to C4 and hydroxy group at C3, is a common molecular backbone for the transfer of multiple long chain fatty acids in the TCA cycle.
Function
editEnergy Generation
editPalmitoylcarnitine is one molecule in a family of ester derivatives of carnitine that are utilized in the TCA cycle to generate energy. The beta oxidation yields 7 NADH, 7 FADH2, and 8 Acetyl-CoA chains. This Acetyl-CoA generates 3 NADH, 1 FADH2, and 1 GTP for every molecule in the TCA cycle. Each NADH generates 2.5 ATP in the ETC and FADH2 generates 1.5 ATP. This totals to 108 ATP, but 2 ATP are consumed to generate the initial Palmitoyl-CoA, leaving a net gain of 106 ATP.[citation needed]
Clinical Significance
editPalmitoylcarnitine has demonstrated potential as a diagnostic marker in newborns for the medical condition of primary carnitine deficiency.[3]
Levels of palmitoylcarnitine (palcar) demonstrated significant correlation with dihydrotestosterone (DHT) and its effects in prostate cancer models, suggesting a similar role between the two molecules.[4]
See also
editReferences
edit- ^ Tein, Ingrid (2015-01-01), Darras, Basil T.; Jones, H. Royden; Ryan, Monique M.; De Vivo, Darryl C. (eds.), "Chapter 40 - Lipid Storage Myopathies Due to Fatty Acid Oxidation Defects", Neuromuscular Disorders of Infancy, Childhood, and Adolescence (Second Edition), San Diego: Academic Press, pp. 761–795, doi:10.1016/b978-0-12-417044-5.00040-8, ISBN 978-0-12-417044-5
- ^ Pande, S. V. (1975-03-01). "A mitochondrial carnitine acylcarnitine translocase system". Proceedings of the National Academy of Sciences. 72 (3): 883–887. Bibcode:1975PNAS...72..883P. doi:10.1073/pnas.72.3.883. ISSN 0027-8424. PMC 432425. PMID 1055387.
- ^ Huang, Y. L.; Tang, C. F.; Liu, S. C.; Sheng, H. Y.; Tang, F.; Jiang, X.; Zheng, R. D.; Mei, H. F.; Liu, L. (2020-06-02). "[Newborn screening for primary carnitine deficiency and variant spectrum of SLC22A5 gene in Guangzhou]". Zhonghua Er Ke Za Zhi = Chinese Journal of Pediatrics. 58 (6): 476–481. doi:10.3760/cma.j.cn112140-20200323-00292. ISSN 0578-1310. PMID 32521959.
- ^ Al‐Bakheit, Ala'a; Traka, Maria; Saha, Shikha; Mithen, Richard; Melchini, Antonietta (2016-10-01). "Accumulation of Palmitoylcarnitine and Its Effect on Pro‐Inflammatory Pathways and Calcium Influx in Prostate Cancer". The Prostate. 76 (14): 1326–1337. doi:10.1002/pros.23222. ISSN 0270-4137. PMC 4996340. PMID 27403764.