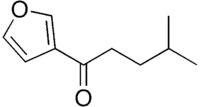
Perilla ketone is a natural terpenoid that consists of a furan ring with a six-carbon side chain containing a ketone functional group. It is a colorless oil that is sensitive to oxygen, becoming colored upon standing. The ketone was identified in 1943 by Sebe as the main component of the essential oil of Perilla frutescens.[1] Perilla ketone is present in the leaves and seeds of purple mint (Perilla frutescens), which is toxic to some animals.[2] When cattle and horses consume purple mint when grazing in fields in which it grows, the perilla ketone causes pulmonary edema leading to a condition sometimes called perilla mint toxicosis.[2]
| |
| Names | |
|---|---|
| Preferred IUPAC name
1-(Furan-3-yl)-4-methylpentan-1-one | |
| Other names
beta-Furyl isoamyl ketone
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C10H14O2 | |
| Molar mass | 166.217 |
| Appearance | Liquid |
| Density | 0.9920 g/cm3 |
| Melting point | <25 °C |
| Boiling point | 196 °C (385 °F; 469 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Synthesis
editPerilla ketone was synthesized in 1957 by Matsuura from 3-furoyl chloride and an organocadmium compound similar to the Gilman reagent made from an isoamyl Grignard reagent and cadmium chloride.[3] Perilla ketone has also been prepared in 74% yield via the Stille reaction from a 3-furyl-organotin compound and isocaproyl chloride in tetrahydrofuran solvent.[4]
See also
editReferences
edit- ^ Sebe, Yeigai (1943). "Supplemental experiments on perilla ketone". Nippon Kagaku Kaishi (in Japanese). 64 (8): 1130–6. doi:10.1246/nikkashi1921.64.1130.
- ^ a b Perilla: Botany, Uses and Genetic Resources
- ^ Matsuura, Teruo (1957). "Natural furan derivatives. I. The synthesis of perilla ketone". Bulletin of the Chemical Society of Japan. 30: 430–1. doi:10.1246/bcsj.30.430.
- ^ Farina, Vittorio; Krishnamurthy, Venkat; Scott, William J. (1997). "The Stille reaction". Organic Reactions. 50: 1–652. doi:10.1002/0471264180.or050.01. ISBN 0471264180.