Plecanatide, sold under the brand name Trulance, is a medication for the treatment of chronic idiopathic constipation (CIC)[3] and irritable bowel syndrome with constipation.[4] It is available in India under the brand name "Plectide" (OQM Div, MSN Laboratories, India). Plecanatide is an agonist of guanylate cyclase-C. Plecanatide increases intestinal transit and fluid through a buildup of cGMP.[5][6]
 | |
| Clinical data | |
|---|---|
| Trade names | Trulance |
| Other names | SP-304 |
| License data |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C65H104N18O26S4 |
| Molar mass | 1681.89 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Chemistry
editPlecanatide, a 16-amino acid peptide structurally identical to human uroguanylin (with exception of a single-amino acid substitution. Plecanatide is a guanylate cyclase-C (GC-C) agonist.
Molecular weight: 1682 Daltons
Structural formula: C65H104N18O26S4
Chemical name: L-Leucine, L-asparaginyl-L-α-aspartyl-L-α-glutamyl-L-cysteinyl-L-αglutamyl-L-leucyl-L-cysteinyl-L-valyl-L-asparaginyl-L-valyl-L-alanyl-L-cysteinyl-L-threonylglycyl-Lcysteinyl-, cyclic (4→12),(7→15)-bis(disulfide).[7]
Medical uses
editAs of January 2017, plecanatide is approved in the United States for the treatment of chronic idiopathic constipation in adults.[3] later it was also approved for irritable bowel syndrome with constipation (IBS-C).[8]
The presence of this condition is determined using the Rome III diagnostic criteria for chronic constipation which requires that the patient meet stool frequency, stool consistency, incomplete evacuation, and straining requirements in addition to not being a likely candidate for irritable bowel syndrome.[4][9] The symptoms should also have been present for at least three of the last six months to establish the chronic nature of the condition before treatment with plecanatide is indicated.[9]
Plecanatide has been shown to be safe and effective. It has shown to be at least equally as effective as its main competitor, linaclotide (brand name Linzess), but has been shown to have a lower rate of diarrhea as an adverse drug reaction.[10]
Dosage and Administration
editThe recommended dosage of plecanatide is 3 mg taken by mouth once daily.[8][11]
A plecanatide tablet can be taken with or without food and should be swallowed whole. For adults with swallowing difficulties, plecanatide tablets can be crushed and administered orally either in apple sauce or with water or administered with water via a nasogastric or gastric feeding tube.[11]
Contraindications
editPlecanatide has not been shown to be safe or effective in persons 6 years to 18 years of age.[12] Use of plecanatide by persons under the age of 6 poses a serious dehydration risk and studies have demonstrated plecanatide can cause death in juvenile mice due to this dehydrating effect.[12]
Use of plecanatide is also contraindicated in persons who are suspected of having a mechanical gastrointestinal obstruction.[12]
Pharmacology
editStructure and function
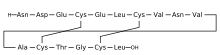
editPlecanatide is a 16 amino acid peptide with the amino acid sequence:
H-Asn1-Asp2-Glu3-Cys4-Glu5-Leu6-Cys7-Val8-Asn9-Val10-Ala11-Cys12-Thr13-Gly14-Cys15-Leu16-OH
(one-letter sequence: NDECELCVNVACTGCL). Plecanatide is nearly structurally identical to human uroguanylin, apart from the substitution of Asp3 with Glu3.[13] Disulfide bonds exist between Cys4 and Cys12, as well as Cys7 and Cys15.[14]
Plecanatide has two important motifs. The first being the acidic residues Asp2 and Glu3 which modulate the affinity for its receptor in response to environmental pH.[12][13][15] Simulations predict the optimal activity of Plecanatide to occur at pH 5, making it suitable for targeting cells within the proximal intestine, which has a pH of between 5 and 6.[12] The second is the ACTGC motif (residues Ala11 to Cys15) which is the region responsible for its binding to the receptor, guanylate cyclase-C.[16]
Mechanism of action
editPlecanatide works as a laxative by drawing water in to the gastrointestinal tract thereby softening stool and encouraging its natural passage.
Similar to its endogenous counterpart, plecanatide activates guanylate cyclase-C on endothelial cells within the gastrointestinal tract.[13] The activation of guanylate cyclase-C catalyses the production of the second messenger guanosine 3’,5’-cyclic monophosphate (cGMP) which leads to the protein kinase A (PKA) and protein kinase G II (PKGII)-mediated phosphorylation of the cystic fibrosis transmembrane conductance regulator (CFTR) protein.[17][18] CFTR is an anion channel and upon activation it will secrete negatively charged ions, particularly chloride (Cl−) and bicarbonate (HCO3−) in to the GI tract lumen.[19][20] This disruption to the electrochemical gradient is in part rectified by the passive secretion of positively charged sodium ions in to the lumen and water follows by osmosis.[19]
Plecanatide is also known to have an anti-nociceptive effect in animal models, however the exact mechanism of action is not yet fully elucidated.[12] It has been suggested that this may be in part to the anti-inflammatory action of guanylate cyclase-C by its inhibition of pro-inflammatory cytokines, or through the inhibition of associated sensory neurons.[21]
Pharmacokinetics and metabolism
editAs plecanatide acts on receptors present on the apical side of endothelial cells lining the gastrointestinal tract it is able to impart its effect without ever entering circulation.[13] As with most orally ingested peptides, plecanatide is degraded by intestinal enzymes, and so very little of the active drug enters systemic circulation.[12] Minimal amounts of the drug are expected to be transported in to the body, and concentrations of plecanatide and its metabolites are undetectable in plasma following the recommended dosage of 3 mg.[12][13] It has also been shown that dosages up to 48.6 mg produced no detectable concentration of plecanatide in human plasma at any time point after ingestion.[13]
References
edit- ^ "FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)". nctr-crs.fda.gov. FDA. Retrieved 22 Oct 2023.
- ^ "Summary Basis of Decision (SBD) for Trulance". Health Canada. 23 October 2014. Retrieved 29 May 2022.
- ^ a b "FDA approves Trulance for Chronic Idiopathic Constipation". FDA.gov. U.S. Food and Drug Administration. Retrieved 20 January 2017.
- ^ a b Miner, Philip B (2020-02-01). "Plecanatide for the treatment of constipation-predominant irritable bowel syndrome". Expert Review of Gastroenterology & Hepatology. 14 (2): 71–84. doi:10.1080/17474124.2020.1722101. ISSN 1747-4124. PMID 31985305. S2CID 210922857.
- ^ "TRULANCE package insert" (PDF). Trulance website. Synergy Pharmaceuticals Inc. 420 Lexington Avenue, Suite 2012 New York, New York 10170. Retrieved 20 January 2017.
- ^ Thomas RH, Luthin DR (June 2015). "Current and emerging treatments for irritable bowel syndrome with constipation and chronic idiopathic constipation: focus on prosecretory agents". Pharmacotherapy. 35 (6): 613–30. doi:10.1002/phar.1594. PMID 26016701. S2CID 24825251.
- ^ "TRULANCE (plecanatide) tablets, for oral use" (PDF). Synergy Pharmaceuticals Inc. U.S. Food and Drug Administration. January 2017.
- ^ a b "TRULANCE (plecanatide) tablets, for oral use" (PDF). Synergy Pharmaceuticals Inc. U.S. Food and Drug Administration. January 2017.
- ^ a b Drossman DA (2006). Rome III: the functional gastrointestinal disorders (3rd ed.). McLean, Va.: Degnon Associates. ISBN 9780965683753. OCLC 79476570.
- ^ "Trulance - FDA prescribing information, side effects and uses". Drugs.com. Retrieved 2017-10-27.
- ^ a b Kamuda JA, Mazzola N (April 2018). "Plecanatide (Trulance) for Chronic Idiopathic Constipation and Irritable Bowel Syndrome With Constipation". P & T: A Peer-Reviewed Journal for Formulary Management. 43 (4): 207–232. PMC 5871240. PMID 29622940.
- ^ a b c d e f g h Al-Salama ZT, Syed YY (April 2017). "Plecanatide: First Global Approval". Drugs. 77 (5): 593–598. doi:10.1007/s40265-017-0718-0. PMID 28255961. S2CID 29735532.
- ^ a b c d e f Shailubhai K, Comiskey S, Foss JA, Feng R, Barrow L, Comer GM, Jacob GS (September 2013). "Plecanatide, an oral guanylate cyclase C agonist acting locally in the gastrointestinal tract, is safe and well-tolerated in single doses". Digestive Diseases and Sciences. 58 (9): 2580–6. doi:10.1007/s10620-013-2684-z. PMID 23625291. S2CID 10123241.
- ^ Chang WL, Masih S, Thadi A, Patwa V, Joshi A, Cooper HS, et al. (February 2017). "+/Min-FCCC mice". World Journal of Gastrointestinal Pharmacology and Therapeutics. 8 (1): 47–59. doi:10.4292/wjgpt.v8.i1.47. PMC 5292606. PMID 28217374.
- ^ Hamra FK, Eber SL, Chin DT, Currie MG, Forte LR (March 1997). "Regulation of intestinal uroguanylin/guanylin receptor-mediated responses by mucosal acidity". Proceedings of the National Academy of Sciences of the United States of America. 94 (6): 2705–10. Bibcode:1997PNAS...94.2705H. doi:10.1073/pnas.94.6.2705. PMC 20153. PMID 9122260.
- ^ Forte LR (November 2004). "Uroguanylin and guanylin peptides: pharmacology and experimental therapeutics". Pharmacology & Therapeutics. 104 (2): 137–62. doi:10.1016/j.pharmthera.2004.08.007. PMID 15518884.
- ^ Hamra FK, Forte LR, Eber SL, Pidhorodeckyj NV, Krause WJ, Freeman RH, et al. (November 1993). "Uroguanylin: structure and activity of a second endogenous peptide that stimulates intestinal guanylate cyclase". Proceedings of the National Academy of Sciences of the United States of America. 90 (22): 10464–8. Bibcode:1993PNAS...9010464H. doi:10.1073/pnas.90.22.10464. PMC 47797. PMID 7902563.
- ^ Bijvelds MJ, Loos M, Bronsveld I, Hellemans A, Bongartz JP, Ver Donck L, et al. (December 2015). "Inhibition of Heat-Stable Toxin-Induced Intestinal Salt and Water Secretion by a Novel Class of Guanylyl Cyclase C Inhibitors". The Journal of Infectious Diseases. 212 (11): 1806–15. doi:10.1093/infdis/jiv300. PMID 25999056.
- ^ a b Gadsby DC, Vergani P, Csanády L (March 2006). "The ABC protein turned chloride channel whose failure causes cystic fibrosis". Nature. 440 (7083): 477–83. Bibcode:2006Natur.440..477G. doi:10.1038/nature04712. PMC 2720541. PMID 16554808.
- ^ Park HW, Nam JH, Kim JY, Namkung W, Yoon JS, Lee JS, et al. (August 2010). "Dynamic regulation of CFTR bicarbonate permeability by [Cl-]i and its role in pancreatic bicarbonate secretion". Gastroenterology. 139 (2): 620–31. doi:10.1053/j.gastro.2010.04.004. PMID 20398666.
- ^ Eutamene H, Bradesi S, Larauche M, Theodorou V, Beaufrand C, Ohning G, et al. (March 2010). "Guanylate cyclase C-mediated antinociceptive effects of linaclotide in rodent models of visceral pain". Neurogastroenterology and Motility. 22 (3): 312–e84. doi:10.1111/j.1365-2982.2009.01385.x. PMID 19706070. S2CID 9190733.
External links
edit- "Plecanatide". Drug Information Portal. U.S. National Library of Medicine.