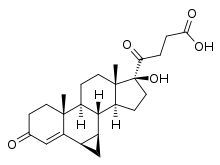
Prorenoic acid, or prorenoate, is a synthetic steroidal antimineralocorticoid which was never marketed.[1][2][3][4]
 | |
| Clinical data | |
|---|---|
| Other names | Prorenoate; 6α,7α-Dihydro-17-hydroxy-3-oxo-3'H-cyclopropa(6,7)-17α-pregna-4,6-diene-21-carboxylic acid |
| Drug class | Antimineralocorticoid |
| Identifiers | |
| |
| CAS Number | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C23H31O4 |
| Molar mass | 371.497 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
See also
editReferences
edit- ^ Elks J (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 1036–. ISBN 978-1-4757-2085-3.
- ^ Claire M, Rafestin-Oblin ME, Michaud A, Roth-Meyer C, Corvol P (April 1979). "Mechanism of action of a new antialdosterone compound, prorenone". Endocrinology. 104 (4): 1194–1200. doi:10.1210/endo-104-4-1194. PMID 436757.
- ^ Netchitailo P, Delarue C, Perroteau I, Jegou S, Tonon MC, Leroux P, et al. (February 1982). "Effect of aldosterone antagonists on mineralocorticoid synthesis in vitro. Inhibition of aldosterone production by prorenoate-K". European Journal of Pharmacology. 77 (4): 243–249. doi:10.1016/0014-2999(82)90125-x. PMID 6277668.
- ^ Morton IK, Hall JM (6 December 2012). Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Science & Business Media. pp. 234–. ISBN 978-94-011-4439-1.