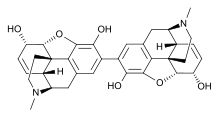
Pseudomorphine (also known as oxydimorphine or dehydromorphine) is an inactive, natural dimerisation product of the morphine molecule in tandem and thus a common impurity in morphine concentrations. It was first described by Pelletier in 1835.[2]
| |
| Names | |
|---|---|
| IUPAC name
(5α,6α)-2-[(5α,6α)-3,6-dihydroxy-17-methyl-7,8-didehydro-4,5-epoxymorphinan-2-yl]-17-methyl-7,8-didehydro-4,5-epoxymorphinan-3,6-diol
| |
| Identifiers | |
3D model (JSmol)
|
|
| Abbreviations | 2,2'-bimorphine[1] |
| ChemSpider | |
| ECHA InfoCard | 100.169.464 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C34H36N2O6 | |
| Molar mass | 568.670 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
This compound may be synthesized by the oxidative coupling of morphine by potassium ferricyanide.[1]
Pseudomorphine contributes very little to morphine's effects. It produces no effects in the central nervous or gastrointestinal systems, but it might have some effects on the circulatory system.[3]
See also
edit- Thebaine (paramorphine)
- Morphine-N-oxide
- Morphine-3-glucuronide
- Morphine-6-glucuronide
References
edit- ^ a b Bentley, K. W.; Dyke, S. F. (1959). "512. The structure of pseudomorphine". Journal of the Chemical Society (Resumed). 1959: 2574–2577. doi:10.1039/JR9590002574.
- ^ A. K. Balls (1927). "Concerning Pseudomorphine". Journal of Biological Chemistry. 71 (2): 537–542. doi:10.1016/S0021-9258(18)84438-6.
- ^ Schmidt, Carl F.; Livingston, A. E. (1933-04-01). "A Note Concerning the Actions of Pseudomorphine". Journal of Pharmacology and Experimental Therapeutics. 47 (4): 473–485. ISSN 0022-3565.