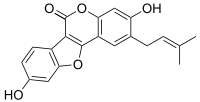
Psoralidin is a natural phenolic compound found in the seeds of Psoralea corylifolia.
| |
| Names | |
|---|---|
| Preferred IUPAC name
3,9-Dihydroxy-2-(3-methylbut-2-en-1-yl)-6H-[1]benzofuro[3,2-c][1]benzopyran-6-one | |
| Other names
3,9-Dihydroxy-2-prenylcoumestan
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.208.688 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C20H16O5 | |
| Molar mass | 336.343 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Chemical synthesis
editPsoralidin production starts with a based catalyzed condensation between phenyl acetate and acid chloride. To form the ring of psoralidin, an intramolecular cyclization occurs, finished off by a microwave assisted cross metathesis reaction.[1]
Pharmacology
editPsoralidin inhibits forskolin-induced corticotrophin releasing factor gene transcription. Recently, it has shown activity in vitro against gastric, colon, prostate, and breast cancer lines. It has the capability to inhibit protein tyrosine phosphatase 1B, a key metabolite involved in insulin signaling.[1]
Psoralidin has shown positive results in the forced swim test, a mouse model of antidepressant activity. Psoralidin raised 5-hydroxytryptamine and 5-hydroxyindoleacetic acid levels in the brain. Dopamine levels changed as well as a result of psoralidin consumption. Stress hormones in mice such as serum corticotropin releasing factor, adrenal corticotropin releasing hormone, and corticosterone were reduced after psoralidin administration.[2]
Structure
editStructurally, psoralidin is a coumestan derivative; it has an isopentenyl group at the second carbon position of coumestrol. Psoralidin is insoluble in water, making in vivo studies difficult.[1]
References
edit- ^ a b c Pahari, Pallab & Rohr, Jürgen (2009). "Total Synthesis of Psoralidin, an Anticancer Natural Product". Journal of Organic Chemistry. 74 (7): 2750–2754. doi:10.1021/jo8025884. PMC 2662043. PMID 19254011.
- ^ Li-Tao Yi; Yu-Cheng Li; Ying Pan; Jian-Mei Li; Qun Xu; Shi-Fu Mo; Chun-Feng Qiao; Fu-Xin Jiang; Hong-Xi Xu; Xiao-Bo Lu; Ling-Dong Kong; Hsiang-Fu Kung (February 2008). "Antidepressant-like effects of psoralidin isolated from the seeds of Psoralea Corylifolia in the forced swimming test in mice". Progress in Neuro-Psychopharmacology & Biological Psychiatry. 32 (2): 510–519. doi:10.1016/j.pnpbp.2007.10.005. PMID 18006202.