Range fractionation is a term used in biology to describe the way by which a group of sensory neurons are able to encode varying magnitudes of a stimulus. Sense organs are usually composed of many sensory receptors measuring the same property. These sensory receptors show a limited degree of precision due to an upper limit in firing rate. If the receptors are endowed with distinct transfer functions in such a way that the points of highest sensitivity are scattered along the axis of the quality being measured, the precision of the sense organ as a whole can be increased.
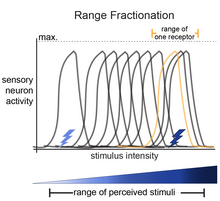
The basis of the idea of range fractionation is that each stimulus (for example, touch) has a range of intensities that can be sensed (light-touch to deep/hard-touch). For an organism to be able to sense a range of stimulus intensities, sensory neurons are tuned to fractions of the entire range. Collectively, the pattern of activity among the sensory neurons is how the organism can identify specific stimulus parameters. This was shown for proprioceptive neurons in the locust leg,[1][2] proprioceptive neurons in the stick insect,[3] Johnston's Organ neurons in Drosophila,[4] and in auditory-sensing neurons in crickets.[5][6][7]
Range fraction is similar to the labeled line theory in that they both describe a phenomenon by which sensory neurons divide the task of encoding a range of stimulus intensities. However the difference lies within the downstream synaptic partners. Labeled line theory describes fully segregated channels postsynaptically. In contrast, sensory neurons that use range fractionation have common synaptic partners, and it is there collective activity that is informative of the stimulus type.
References
edit- ^ Usherwood PN, Runion HI, Campbell JI (1968). "Structure and physiology of a chordotonal organ in the locust leg". Journal of Experimental Biology. 48 (2): 305–323. doi:10.1242/jeb.48.2.305.
- ^ Matheson T (1992). "Range fractionation in the locust metathoracic femoral chordotonal organ". Journal of Comparative Physiology A. 170 (4): 509–520. doi:10.1007/BF00191466. S2CID 26197182.
- ^ Hofmann T, Koch UT, Bassler U (1985-01-01). "Physiology of the Femoral Chordotonal Organ in the Stick Insect, Cuniculina Impigra". Journal of Experimental Biology. 114 (1): 207–223. doi:10.1242/jeb.114.1.207. ISSN 1477-9145.
- ^ Patella P, Wilson RI (April 2018). "Functional Maps of Mechanosensory Features in the Drosophila Brain". Current Biology. 28 (8): 1189–1203.e5. doi:10.1016/j.cub.2018.02.074. PMC 5952606. PMID 29657118.
- ^ Oldfield BP (1983). "Central projections of primary auditory fibres in Tettigoniidae (Orthoptera: Ensifera)". Journal of Comparative Physiology A. 151 (3): 389–395. doi:10.1007/bf00623914. ISSN 0340-7594. S2CID 11430039.
- ^ Shimozawa T, Kanou M (1984). "Varieties of filiform hairs: range fractionation by sensory afferents and cereal interneurons of a cricket". Journal of Comparative Physiology A. 155 (4): 485–493. doi:10.1007/bf00611913. ISSN 0340-7594. S2CID 42806812.
- ^ Oldfield BP, Kleindienst HU, Huber F (October 1986). "Physiology and tonotopic organization of auditory receptors in the cricket Gryllus bimaculatus DeGeer". Journal of Comparative Physiology A. 159 (4): 457–464. doi:10.1007/bf00604165. PMID 3783498. S2CID 27321719.