Razupenem (PTZ-601) is a broad spectrum injectable antibiotic, from the carbapenem subgroup of beta-lactam antibiotics. It was developed as a replacement drug to combat bacteria that had acquired antibiotic resistance to commonly used antibiotics.[1] Razupenem performed well against a variety of bacterial strains,[2] but further development is in doubt due to a high rate of side effects in Phase II clinical trials.
 | |
| Clinical data | |
|---|---|
| Trade names | PTZ-601 |
| Routes of administration | IV |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| ChemSpider | |
| UNII | |
| KEGG | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
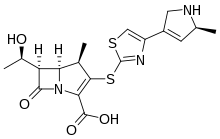
| Formula | C18H21N3O4S2 |
| Molar mass | 407.50 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
References
edit- ^ Livermore DM, Mushtaq S, Warner M (August 2009). "Activity of the anti-MRSA carbapenem razupenem (PTZ601) against Enterobacteriaceae with defined resistance mechanisms". The Journal of Antimicrobial Chemotherapy. 64 (2): 330–5. doi:10.1093/jac/dkp187. PMID 19497942.
- ^ Tran CM, Tanaka K, Yamagishi Y, Goto T, Mikamo H, Watanabe K (May 2011). "In vitro antimicrobial activity of razupenem (SMP-601, PTZ601) against anaerobic bacteria". Antimicrobial Agents and Chemotherapy. 55 (5): 2398–402. doi:10.1128/AAC.01038-10. PMC 3088197. PMID 21343447.