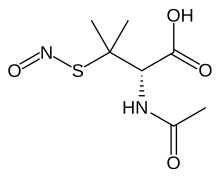
S-Nitroso-N-acetylpenicillamine (SNAP) is the organosulfur compound with the formula ONSC(CH3)2CH(NHAc)CO2H. It is a green solid.[2]
| |

| |
| Names | |
|---|---|
| IUPAC name
S-Nitroso-N-acetylpenicillamine
| |
| Other names
N-Acetyl-3-(nitrosothio)-DL-valine
S-Nitroso-N-acetylpenicillamine | |
| Identifiers | |
3D model (JSmol)
|
|
| Abbreviations | SNAP |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C7H12N2O4S | |
| Molar mass | 220.25 g/mol |
| Appearance | green solid |
| Hazards | |
| GHS labelling:[1] | |

| |
| Warning | |
| H315, H319, H335 | |
| P261, P264, P271, P280, P302+P352, P305+P351+P338 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
SNAP is an S-nitrosothiol and is used as a model for the general class of S-nitrosothiols which have received much attention in biochemistry because nitric oxide and some organic nitroso derivatives serve as signaling molecules in living systems, especially related to vasodilation.[3] SNAP is derived from the amino acid penicillamine. S-Nitrosoglutathione is a related agent.
References
edit- ^ "N3398 h S-Nitroso-N-acetyl-DL-penicillamine". Sigma-Aldric. Retrieved 13 December 2021.
- ^ Arulsamy, N.; Bohle, D. S.; Butt, J. A.; Irvine, G. J.; Jordan, P. A.; Sagan, E. (1999). "Interrelationships between Conformational Dynamics and the Redox Chemistry of S-Nitrosothiols". Journal of the American Chemical Society. 121 (30): 7115–7123. doi:10.1021/ja9901314.
- ^ Zhang Y.; Hogg, N. (2005). "S-Nitrosothiols: Cellular Formation and Transport". Free Radical Biology and Medicine. 38 (7): 831–838. doi:10.1016/j.freeradbiomed.2004.12.016. PMID 15749378.