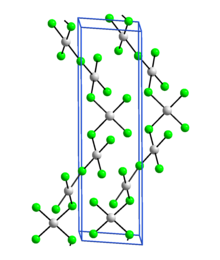
Silver(III) fluoride, AgF3, is an unstable, bright-red, diamagnetic compound containing silver in the unusual +3 oxidation state. Its crystal structure is very similar to that of gold(III) fluoride: it is a polymer consisting of rectangular AgF4 units linked into chains by fluoro bridges.[1]
| |
| Names | |
|---|---|
| IUPAC name
Silver(III) fluoride
| |
| Other names
Silver trifluoride
Argentic fluoride | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| 100808 | |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| AgF3 | |
| Molar mass | 164.86 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Preparation
editAgF3 can be prepared by treating a solution containing tetrafluoroargentate(III) ions in anhydrous hydrogen fluoride with boron trifluoride;[2] the potassium tetrafluoroargentate(III) was prepared by heating a stoichiometric mix of potassium and silver nitrate in a sealed container filled with pressurised fluorine gas at 400C for 24 hours, twice. When dissolved in anhydrous HF, it decomposes spontaneously to Ag3F8 overnight at room temperature. The high-valence silver compounds described in the thesis are notable for their variety of colours: KAgF4 is bright orange, AgF3 bright red, AgFAsF6 is deep blue, Ag3F8 deep red-brown, and Pd(AgF4)2 is lime-green.
Earlier preparations used krypton difluoride as fluorinating agent, and tended to produce the mixed-valence Ag3F8 which may be thought of as silver(II) tetrafluoroargentate(III); Ag2F5, which is (AgF)+AgF4−, is formed by reacting AgF3 with AgFAsF6.
References
edit- ^ Zemva, Boris; Lutar, Karel; Jesih, Adolf; Casteel, William J.; Wilkinson, Angus P.; Cox, David E.; von Dreele, Robert B.; Borrmann, Horst; Bartlett, Neil (1991). "Silver Trifluoride: Preparation, Crystal Structure, Some Properties, and Comparison with AuF3". J Am Chem Soc. 113 (11): 4192–4198. doi:10.1021/ja00011a021.
- ^ Casteel, William (September 1992). The Synthesis and Structural Characterization of Novel Transition Metal Fluorides (PhD).