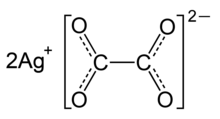
Silver oxalate (Ag
2C
2O
4) is a silver salt of oxalic acid commonly employed in experimental petrology to add carbon dioxide (CO
2) to experiments as it will break down to silver (Ag) and carbon dioxide under geologic conditions.[2] It is also a precursor to the production of silver nanoparticles.
It is explosive upon heating around 140 degrees Celsius, shock or friction.
[3]
| |
| Names | |
|---|---|
| IUPAC name
Silver(I) ethanedioate
| |
| Other names
Silver Ethanedioate, Silver Salt
Argentous oxalate Silver(I) oxalate | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.007.791 |
| EC Number |
|
PubChem CID
|
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| Ag 2C 2O 4 | |
| Molar mass | 303.755 g/mol |
| Appearance | white powder |
| Density | 5.03 g/cm3 |
| Melting point | 961.9 °C (1,763.4 °F; 1,235.0 K) (decomposes) |
| Boiling point | 2,212 °C (4,014 °F; 2,485 K) at 1013.25 hPa |
| 3.270*10−3 g/100mL | |
Solubility product (Ksp)
|
5.4×10−12[1] |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
Harmful if swallowed |
| Safety data sheet (SDS) | External MSDS |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Production
editSilver oxalate is produced by the reaction between silver nitrate and oxalic acid.
See also
editReferences
edit- ^ John Rumble (June 18, 2018). CRC Handbook of Chemistry and Physics (99 ed.). CRC Press. pp. 5–189. ISBN 978-1138561632.
- ^ Silver Oxalate at American Elements
- ^ Silver Oxalate MSDS sheet Archived 2013-12-12 at the Wayback Machine at mpbio