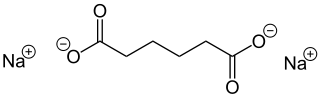
Sodium adipate is a chemical organic compound with formula Na2C6H8O4. It is the sodium salt of adipic acid.
| |
| Names | |
|---|---|
| Preferred IUPAC name
Disodium hexanedioate | |
| Other names
Disodium adipate
Adipic acid, sodium salt | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.028.448 |
| E number | E356 (antioxidants, ...) |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C6H8Na2O4 | |
| Molar mass | 190.106 g·mol−1 |
| Appearance | Solid white to off-white powder or crystals |
| Hazards | |
| GHS labelling: | |

| |
| Warning | |
| H302, H315, H319, H335 | |
| P261, P305+P351+P338 | |
| NFPA 704 (fire diamond) | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
4000 mg/kg (intraperitoneal, mouse) |
| Safety data sheet (SDS) | [1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
As a food additive, it has the E number E356 as is used as a buffering agent and as an acidity regulator.[1]
Preparation
editSodium adipate is prepared by reacting adipic acid with sodium hydroxide:[2]
Safety
editIf consumed in excess, it can lead to high levels of sodium and gastrointestinal problems. It can also cause allergic reactions which may lead to swelling, itching, difficulty breathing. Sodium adipate has no proven health benefits.
References
edit- ^ "E356 Sodium adipate". food-info.net.
- ^ "Sodium Adipate (E356) – Overview, Uses, Side Effects & More". healthknight.com.
