Sodium decavanadate describes any member of the family of inorganic compounds with the formula Na6[V10O28](H2O)n. These are sodium salts of the orange-colored decavanadate anion [V10O28]6−.[1] Numerous other decavanadate salts have been isolated and studied since 1956 when it was first characterized.[2]
| |

| |
| Identifiers | |
|---|---|
| |
3D model (JSmol)
|
|
| ChemSpider |
|
| EC Number |
|
PubChem CID
|
|
| |
| |
| Properties | |
| Na6[V10O28] | |
| Molar mass | 1419.6 g/mol |
| Appearance | orange solid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Preparation
editThe preparation of decavanadate is achieved by acidifying an aqueous solution of ortho-vanadate:[1]
- 10 Na3[VO4] + 24 HOAc → Na6[V10O28] + 12 H2O + 24 NaOAc
The formation of decavanadate is optimized by maintaining a pH range of 4–7. Typical side products include metavanadate, [VO3]−, and hexavanadate, [V6O16]2−, ions.[1]
Structure
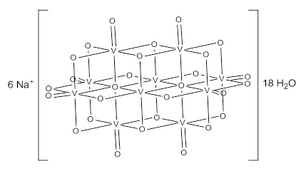
editThe decavanadate ion consists of 10 fused VO6 octahedra and has D2h symmetry.[3][4][5] The structure of Na6[V10O28]·18H2O has been confirmed with X-ray crystallography.[6]
The decavanadate anions contains three sets of equivalent V atoms (see fig. 1).[3] These include two central VO6 octahedra (Vc) and four each peripheral tetragonal-pyramidal VO5 groups (Va and Vb). There are seven unique groups of oxygen atoms (labeled A through G). Two of these (A) bridge to six V centers, four (B) bridge three V centers, fourteen of these (C, D and E) span edges between pairs of V centers, and eight (F and G) are peripheral.
The oxidation state of vanadium in decavanadate is +5.
Acid-base properties
editAqueous vanadate (V) compounds undergo various self-condensation reactions.[7] Depending on pH, major vanadate anions in solution include VO2(H2O)42+, VO43−, V2O73−, V3O93−, V4O124−, and V10O286−. The anions often reversibly protonate.[5] Decavanadate forms according to this equilibrium:[2][7]
- H3V10O283− ⇌ H2V10O284− + H+
- H2V10O284− ⇌ HV10O285− + H+
- HV10O285−(aq) ⇌ V10O286− + H+
The structure of the various protonation states of the decavanadate ion has been examined by 51V NMR spectroscopy.[5][7] Each species gives three signals; with slightly varying chemical shifts around −425, −506, and −523 ppm relative to vanadium oxytrichloride; suggesting that rapid proton exchange occurs resulting in equally symmetric species.[8] The three protonations of decavanadate have been shown to occur at the bridging oxygen centers, indicated as B and C in figure 1.[8]
Decavanadate is most stable in pH 4–7 region.[1][4][7] Solutions of vanadate turn bright orange at pH 6.5, indicating the presence of decavanadate. Other vanadates are colorless. Below pH 2.0, brown V2O5 precipitates as the hydrate.[3][7]
- V10O286− + 6H+ + 12H2 ⇌ 5V2O5
Potential uses
editDecavanadate has been found to inhibit phosphoglycerate mutase, an enzyme which catalyzes step 8 of glycolysis. In addition, decavandate was found to have modest inhibition of Leishmania tarentolae viability, suggesting that decavandate may have a potential use as a topical inhibitor of protozoan parasites.[9]
Related decavanadates
editMany decavanadate salts have been characterized. NH4+, Ca2+, Ba2+, Sr2+, and group I decavanadate salts are prepared by the acid-base reaction between V2O5 and the oxide, hydroxide, carbonate, or hydrogen carbonate of the desired positive ion.[1]
- 6 NH3 + 5 V2O5 + 3 H2O ⇌ (NH4)6[V10O28]
Other decavanadates:
- (NH4)6[V10O28]·6H2O[2]
- K6[V10O28]·9H2O[2]
- K6[V10O28]·10H2O[1][2][3]
- Ca3[V10O28]·16H2O[2][3]
- K2Mg2[V10O28]·16H2O[2][3]
- K2Zn2[V10O28]·16H2O[1][2][3]
- Cs2Mg2[V10O28]·16H2O[3]
- Cs4Na2[V10O28]·10H2O[10]
- K4Na2[V10O28]·16H2O[11]
- Sr3[V10O28]·22H2O[10]
- Ba3[V10O28]·19H2O[10]
- [(C6H5)4P]H3V10O28·4CH3CN[8]
- Ag6[V10O28]·4H2O[12][13]
Naturally occurring decavanadates include:
- Ca3V10O28·17 H2O (Pascoite)
- Ca2Mg(V10O28)·16H2O (Magnesiopascoite)
- Na4Mg(V10O28)·24H2O (Huemulite)
References
edit- ^ a b c d e f g Johnson, G.; Murmann, R. K. (1979). "Sodium and Ammonium Decayanadates(V)". Inorganic Syntheses. Vol. 19. pp. 140–145. doi:10.1002/9780470132500.ch32. ISBN 978-0-471-04542-7.
- ^ a b c d e f g h Rossotti, F. J.; Rossotti, H. (1956). "Equilibrium Studies of Polyanions". Acta Chemica Scandinavica. 10: 957–984. doi:10.3891/acta.chem.scand.10-0957.
- ^ a b c d e f g h Evans, H. T. Jr (1966). "The molecular structure of the isopoly complex ion, decavanadate". Inorg. Chem. 5: 967–977. doi:10.1021/ic50040a004.
- ^ a b Kustin, K.; Pessoa, J. C.; Crans, D. C. (2007). Vandadium: The Versatile Metal. Washington, D. C.: American Chemical Society. ISBN 978-0-8412-7446-4.
- ^ a b c Rehder, D. (2008). Bioinorganic Vanadium Chemistry. Wiley & Sons. pp. 13–51. ISBN 978-0-470-06509-9.
- ^ Durif, P.A.; Averbuch-pouchot, M.T. (1980). "Structure d'un Décavanadate d'Hexasodium Hydraté". Acta Crystallogr. B. 36 (3): 680–682. Bibcode:1980AcCrB..36..680D. doi:10.1107/S0567740880004116.
- ^ a b c d e Tracey, A.S.; Crans, D.C. (1998). Vanadium Compounds. Washington D.C.: American Chemical Society. ISBN 0-8412-3589-9.
- ^ a b c Day, V. W.; Klemperer, W. G.; Maltbie, D. J. (1987). "Where Are the Protons in H3V10O283−?". Journal of the American Chemical Society. 109 (10): 2991–3002. doi:10.1021/ja00244a022.
- ^ Turner, Timothy; Nguyen, Victoria; McLauchlan, Craig; Dymon, Zaneta; Dorsey, Benjamin; Hooker, Jaqueline; Jones, Marjorie (March 2012). "Inhibitory effects of decavanadate on several enzymes and Leishmania tarentolae In Vitro". Journal of Inorganic Biochemistry. 108: 96–104. doi:10.1016/j.jinorgbio.2011.09.009. PMID 22005446. Retrieved 23 January 2021.
- ^ a b c Dametto, A.C.; de Arauju, A.S.; de Souza Correa, R.; Guilherme, L.R.; Massabni, A.C. (2010). "Synthesis, infrared spectroscopy and crystal structure determination of a new decavanadate". J Chem Crystallogr. 40 (11): 897–901. doi:10.1007/s10870-010-9759-x. S2CID 97736357.
- ^ Matias, P.M.; Pessoa, J.C.; Duarte, M.T.; Maderia, C. (2000). "Tetrapotassium disodium decavanadate(V) decahydrate". Acta Crystallogr. C. 57 (3): e75–e76. Bibcode:2000AcCrC..56E..75M. doi:10.1107/S0108270100001530. PMID 15263200.
- ^ Escobar, M.E.; Baran, E.J. (1981). "Die Schwingungsspektren einiger kristalliner Dekavanadate". Monatshefte für Chemie. 112: 43–49. doi:10.1007/BF00906241. S2CID 101366009.
- ^ Aureliano, Manuel; Crans, Debbie C. (2009). "Decavanadate (V
10O6−
28) and oxovanadates: Oxometalates with many biological activities". Journal of Inorganic Biochemistry. 103 (4): 536–546. doi:10.1016/j.jinorgbio.2008.11.010. ISSN 0162-0134. PMID 19110314.