This article is missing information about production and uses. (September 2024) |
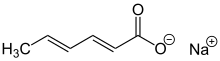
Sodium sorbate is the sodium salt of sorbic acid. It is an unstable white solid. Unlike other sorbic acid salts such as potassium sorbate (E202) and calcium sorbate (E203), the use of sodium sorbate as a food additive is prohibited in the EU due to potentially genotoxic effects.[2][3]
| |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Sodium (2E,4E)-hexa-2,4-dienoate | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
| ECHA InfoCard | 100.028.927 | ||
| E number | E201 (preservatives) | ||
PubChem CID
|
|||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C6H7NaO2 | |||
| Molar mass | 134.10835 g/mol | ||
| Odor | hydrocarbon-like | ||
| Boiling point | 233 °C (451 °F; 506 K)[1] | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Its E-number is E201.
References
edit- ^ Datenbankeintrag bei Chemspider
- ^ EFSA Journal 2015
- ^ Erich Lück, Martin Jager, Nico Raczek (2000). "Sorbic Acid". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a24_507. ISBN 3527306730.
{{cite encyclopedia}}: CS1 maint: multiple names: authors list (link)

