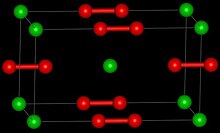
Strontium peroxide is an inorganic compound with the formula Sr O2 that exists in both anhydrous and octahydrate form, both of which are white solids. The anhydrous form adopts a structure similar to that of calcium carbide.[4][5]
| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| ECHA InfoCard | 100.013.841 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| SrO2 | |
| Molar mass | 119.619 g/mol |
| Appearance | white powder |
| Odor | odorless |
| Density | 4.56 g/cm3 (anhydrous) 1.91 g/cm3 (octahydrate) |
| Melting point | 215 °C (419 °F; 488 K) (decomposes)[1] |
| slightly soluble | |
| Solubility | very soluble in alcohol, ammonium chloride insoluble in acetone |
| Structure | |
| Tetragonal [2] | |
| D174h, I4/mmm, tI6 | |
| 6 | |
| Hazards | |
| GHS labelling: | |
   [3] [3]
| |
| Danger | |
| H302, H312, H317, H331, H350 | |
| P220, P261, P280, P305+P351+P338 | |
| Safety data sheet (SDS) | External SDS |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Uses
editIt is an oxidizing agent used for bleaching. It is used in some pyrotechnic compositions as an oxidizer and a vivid red pyrotechnic colorant. It can also be used as an antiseptic and in tracer munitions.[citation needed]
Production
editStrontium peroxide is produced by passing oxygen over heated strontium oxide. Upon heating in the absence of O2, it degrades to SrO and O2. It is more thermally labile than BaO2.[6][7]
References
edit- ^ Middleburgh, Simon C.; Lagerlof, Karl Peter D.; Grimes, Robin W. (2013). "Accommodation of Excess Oxygen in Group II Monoxides". Journal of the American Ceramic Society. 96: 308–311. doi:10.1111/j.1551-2916.2012.05452.x.
- ^ Massalimov, I. A.; Kireeva, M. S.; Sangalov, Yu. A. (2002). "Structure and Properties of Mechanically Activated Barium Peroxide". Inorganic Materials. 38 (4): 363–366. doi:10.1023/A:1015105922260. S2CID 91881752.
- ^ "Strontium Peroxide". American Elements. Retrieved March 7, 2019.
- ^ Bernal, J. D.; D'yatlova, E.; Kasarnovskii, I.; Raikhstein, S. I.; Ward, A. G. "The structure of strontium and barium peroxides" Zeitschrift für Kristallographie, Kristallgeometrie, Kristallphysik, Kristallchemie (1935), 92, 344-54.
- ^ Natta, G. "Structure of hydroxides and hydrates. IV. Octahydrated strontium peroxide" Gazzetta Chimica Italiana (1932), 62, 444-56.
- ^ Middleburgh, Simon C.; Lagerlof, Karl Peter D.; Grimes, Robin W. (2013). "Accommodation of Excess Oxygen in Group II Monoxides". Journal of the American Ceramic Society. 96: 308–311. doi:10.1111/j.1551-2916.2012.05452.x.
- ^ Bauschlicher, Charles W. Jr.; Partridge, Harry; Sodupe, Mariona; Langhoff, Stephen R. "Theoretical study of the alkaline-earth metal superoxides BeO2 through SrO2" Journal of Physical Chemistry 1992, volume 96, pp. 9259-64. doi:10.1021/j100202a036