Talsupram (Lu 5-005 or Lu 5-003[1]) is a selective norepinephrine reuptake inhibitor (NRI) which was investigated as an antidepressant in the 1960s and 1970s but was never marketed.[2][3][4] Along with talopram, it is structurally related to the selective serotonin reuptake inhibitor (SSRI) citalopram.[5]
 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
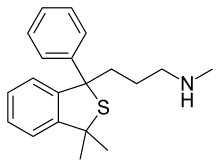
| Formula | C20H25NS |
| Molar mass | 311.49 g·mol−1 |
See also
editReferences
edit- ^ Carlsson A, Fuxe K, Hamberger B, Malmfors T (May 1969). "Effect of a new series of bicyclic compounds with potential thymoleptic properties on the reserpine-resistant uptake mechanism of central and peripheral monoamine neurones in vivo and in vitro". British Journal of Pharmacology. 36 (1): 18–28. doi:10.1111/j.1476-5381.1969.tb08299.x. OCLC 6895508396. PMC 1703539. PMID 5768100.
- ^ Pawłowski L, Mazela H (June 1986). "Effects of antidepressant drugs, selective noradrenaline-or 5-hydroxytryptamine uptake inhibitors, on apomorphine-induced hypothermia in mice". Psychopharmacology. 88 (2): 240–246. doi:10.1007/BF00652248. OCLC 5653283278. PMID 3006113. S2CID 1732297.
- ^ McConathy J, Owens MJ, Kilts CD, Malveaux EJ, Camp VM, Votaw JR, et al. (August 2004). "Synthesis and biological evaluation of [11C]talopram and [11C]talsupram: candidate PET ligands for the norepinephrine transporter". Nuclear Medicine and Biology. 31 (6): 705–718. doi:10.1016/j.nucmedbio.2003.05.001. PMID 15246361.
- ^ Kelliher P, Kelly JP, Leonard BE, Sánchez C (April 2003). "Effects of acute and chronic administration of selective monoamine re-uptake inhibitors in the rat forced swim test". Psychoneuroendocrinology. 28 (3): 332–347. doi:10.1016/S0306-4530(02)00026-4. PMID 12573300. S2CID 23452713.
- ^ "The SSRI Issues" (DOC). healyprozac.com. Retrieved January 3, 2024.