Tantalum(V) chloride, also known as tantalum pentachloride, is an inorganic compound with the formula TaCl5. It takes the form of a white powder and is commonly used as a starting material in tantalum chemistry. It readily hydrolyzes to form tantalum(V) oxychloride (TaOCl3) and eventually tantalum pentoxide (Ta2O5); this requires that it be synthesised and manipulated under anhydrous conditions, using air-free techniques.
| |
| Names | |
|---|---|
| IUPAC names
Tantalum(V) chloride
Tantalum pentachloride | |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.028.869 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| TaCl5 | |
| Molar mass | 358.213 g/mol |
| Appearance | white monoclinic crystals[1] |
| Density | 3.68 g/cm3 |
| Melting point | 216 °C (421 °F; 489 K) |
| Boiling point | 239.4 °C (462.9 °F; 512.5 K) (decomposes) |
| reacts | |
| Solubility | chloroform, CCl4 |
| +140.0×10−6 cm3/mol | |
| Structure | |
| Monoclinic, mS72 | |
| C2/m, No. 12 | |
| Thermochemistry | |
Std molar
entropy (S⦵298) |
221.75 J K−1 mol−1 |
Std enthalpy of
formation (ΔfH⦵298) |
-858.98 kJ/mol |
| Hazards | |
| GHS labelling: | |
 
| |
| Danger | |
| H302, H314 | |
| P280, P305+P351+P338, P310 | |
| NFPA 704 (fire diamond) | |
| Flash point | Non-flammable |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
1900 mg/kg (oral, rat) |
| Safety data sheet (SDS) | External SDS |
| Related compounds | |
Other anions
|
Tantalum(V) fluoride Tantalum(V) bromide Tantalum(V) iodide |
Other cations
|
Vanadium(IV) chloride Niobium(V) chloride |
Related compounds
|
Tantalum(III) chloride, Tantalum(IV) chloride |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
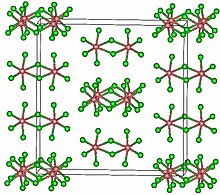
Structure
editTaCl5 crystallizes in the monoclinic space group C2/m.[2] The ten chlorine atoms define a pair of octahedra that share a common edge. The tantalum atoms occupy the centres of the octahedra and are joined by two chlorine bridging ligands. The dimeric structure is retained in non-complexing solvents and to a large extent in the molten state. In the vapour state, however, TaCl5 is monomeric. This monomer adopts a trigonal bipyramidal structure, like that of PCl5.[3]
Physical Properties
editThe solubility of tantalum pentachloride increases slightly for the following series of aromatic hydrocarbons:
- benzene < toluene < m-xylene < mesitylene
This is reflected in the deepening of colour of the solutions from pale yellow to orange. Tantalum pentachloride is less soluble in cyclohexane and carbon tetrachloride than in the aromatic hydrocarbons. Such solutions of tantalum pentachloride are also known to be poor conductors of electricity, indicating little ionization. TaCl5 is purified by sublimation to give white needles.
Synthesis
editTantalum pentachloride can be prepared by reacting powdered metallic tantalum with chlorine gas at between 170 and 250 °C. This reaction can also be performed using HCl at 400 °C.[4]
- 2 Ta + 5 Cl2 → 2 TaCl5
- 2 Ta + 10 HCl → 2 TaCl5 + 5 H2
It can also be prepared by a reaction between tantalum pentoxide and thionyl chloride at 240 °C
- Ta2O5 + 5 SOCl2 → 2 TaCl5 + 5 SO2
Tantalum pentachloride is commercially available, however samples can be contaminated with tantalum(V) oxychloride (TaOCl3), formed by hydrolysis.
Reactions
editTaCl5 is electrophilic and it behaves like a Friedel–Crafts catalyst, similar to AlCl3. It forms adducts with a variety of Lewis bases.[5]
Simple adducts
editTaCl5 forms stable complexes with ethers:
- TaCl5 + R2O → TaCl5(OR2) (R = Me, Et)
TaCl5 also reacts with phosphorus pentachloride and phosphorus oxychloride, the former as a chloride donor and the latter serves as a ligand, binding through the oxygen:
- TaCl5 + PCl5 → [PCl+
4][TaCl−
6] - TaCl5 + OPCl3 → [TaCl5(OPCl3)]
Tantalum pentachloride reacts with tertiary amines to give crystalline adducts.
- TaCl5 + 2 R3N → [TaCl5(NR3)]
Chloride displacement reactions
editTantalum pentachloride reacts at room temperature with an excess of triphenylphosphine oxide to give oxychlorides:
- TaCl5 + 3 OPPh3 → [TaOCl3(OPPh3)]x ...
The presumed initial formation of adducts between TaCl5 and hydroxyl compounds such as alcohols, phenols and carboxylic acids is followed immediately by the elimination of hydrogen chloride and the formation of Ta–O bonds:
- TaCl5 + 3 HOEt → TaCl2(OEt)3 + 3 HCl
In the presence of ammonia as a HCl acceptor, all five chloride ligands are displaced with formation of Ta(OEt)5. Similarly TaCl5 reacts with lithium methoxide in anhydrous methanol to form related methoxy derivatives:
- TaCl5 + 4 LiOMe → Ta(OMe)4Cl + 4 LiCl
Ammonolysis and alcoholysis and related reactions
editAmmonia will displace most of the chloride ligands from TaCl5 to give a cluster. Chloride is displaced more slowly by primary or secondary amines but the replacement of all five chloride centers by amido groups has been achieved by the use of lithium dialkylamides, as illustrated by the synthesis of pentakis(dimethylamido)tantalum:
- TaCl5 + 5 LiNMe2 → Ta(NMe2)5
With alcohols, the pentachloride reacts to give alkoxides. As shown for the preparation of tantalum(V) ethoxide, such reactions are often conducted in the presence of base:
- 10 EtOH + Ta2Cl10 + 10 NH3 → Ta2(OEt)10 + 10 NH4Cl
Tantalum pentachloride is reduced by nitrogen heterocycles such as pyridine.
Reduction
editReduction of tantalum(V) chloride gives anionic and neutral clusters including [Ta6Cl18]4− and [Ta6Cl14](H2O)4.[6]
References
edit- ^ Lide, David R., ed. (2006). CRC Handbook of Chemistry and Physics (87th ed.). Boca Raton, FL: CRC Press. ISBN 0-8493-0487-3.
- ^ Rabe, Susanne; Müller, Ulrich (2000). "Crystal structure of tantalum pentachloride, (TaCl5)2". Z. Kristallogr. - New Cryst. Struct. 215 (1): 1–2. doi:10.1515/ncrs-2000-0102.
- ^ F. Fairbrother (1967). The Chemistry of Niobium and Tantalum. Elsevier.
- ^ Young, Ralph C.; Brubaker, Carl H. (1952). "Reaction of Tantalum with Hydrogen Chloride, Hydrogen Bromide and Tantalum Pentachloride; Action of Hydrogen on Tantalum Pentachloride". Journal of the American Chemical Society. 74 (19): 4967. doi:10.1021/ja01139a524.
- ^ F. A. Cotton, G. Wilkinson, Advanced Inorganic Chemistry (4th ed.), Wiley, New York, 1980.
- ^ Duraisamy, Thirumalai; Hay, Daniel N. T.; Messerle, Louis (2014). "Octahedral Hexatantalum Halide Clusters". Inorganic Syntheses: Volume 36. Vol. 36. pp. 1–8. doi:10.1002/9781118744994.ch1. ISBN 9781118744994.
- ^ Thaxton, C. B.; Jacobson, R. A. (1971). "The Crystal Structure of H2(Ta6Cl18)(H2O)6". Inorganic Chemistry. 10: 1460–1463. doi:10.1021/ic50101a029.
Further reading
edit- Ozin, G. A.; Walton, R. A. (1970). "Vibrational spectra and structures of the 1:1 complexes of niobium and tantalum, pentachlorides and tantalum pentabromide with aceto-, perdeuterioaceto-, and propionitriles in the solid and solution states and a vibrational analysis of the species MX5, NC·CY3 (Y = H or D)". J. Chem. Soc. A: 2236–2239. doi:10.1039/j19700002236.
- Bullock, J. I.; Parrett, F. W.; Taylor, N. J. (1973). "Some metal halide–phosphorus halide–alkyl halide complexes. Part II. Reactions with niobium and tantalum pentachlorides and tungsten hexachloride". J. Chem. Soc., Dalton Trans. (5): 522–524. doi:10.1039/DT9730000522.
- Đorđević, C.; Katović, V. (1970). "Co-ordination complexes of niobium and tantalum. Part VIII. Complexes of niobium(IV), niobium(V), and tantalum(V) with mixed oxo, halogeno, alkoxy, and 2,2′-bipyridyl ligands". J. Chem. Soc. A: 3382–3386. doi:10.1039/j19700003382.
- Cowley, A.; Fairbrother, F.; Scott, N. (1958). "The halides of niobium (columbium) and tantalum. Part V. Diethyl ether complexes of the pentachlorides and pentabromides; the solubility of tantalum pentaiodide in ether". J. Chem. Soc.: 3133–3137. doi:10.1039/JR9580003133.
