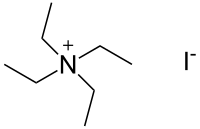
Tetraethylammonium iodide is a quaternary ammonium compound with the chemical formula C8H20N+I−. It has been used as the source of tetraethylammonium ions in pharmacological and physiological studies, but is also used in organic chemical synthesis.
| |
| Names | |
|---|---|
| Preferred IUPAC name
N,N,N-Triethylethanaminium iodide | |
| Other names
Tetamon iodide; Tetramon J; TEAI
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.000.615 |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C8H20IN | |
| Molar mass | 257.159 g·mol−1 |
| Appearance | Colorless or yellowish crystalline solid |
| Density | 1.566 g/cm3[1] |
| Melting point | 280 °C (536 °F; 553 K) (decomposes) |
| soluble | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Chemistry
editPreparation
editTetraethylammonium iodide is commercially available, but can be prepared by the reaction between triethylamine and ethyl iodide.[2]
Structure
editThe crystal structure of tetraethylammonium iodide has been determined.[3] The crystal structure is a distorted wurtzite lattice. At the nitrogen atom, the coordination is a flattened tetrahedron. The N−C−C angle is slightly larger than the tetrahedral angle.
Synthetic applications
editExamples include:
- Stereoselective formation of (Z)-diiodoalkenes by treatment of alkynes with ICl in the presence of tetraethylammonium iodide.[4]
- 2-Hydroxyethylation (attachment of −CH2−CH2−OH) by ethylene carbonate of carboxylic acids and certain heterocycles bearing an acidic N-H. For example, benzoic acid is converted to the ester, 2-hydroxyethyl benzoate, by treatment with ethylene carbonate in the presence of tetraethylammonium iodide.[5]
- Phase-transfer catalyst in geminal di-alkylation of fluorene, N,N-dialkylation of aniline and N-alkylation of carbazole using aqueous sodium hydroxide and alkyl halides.[6]
Toxicity
editLD50: 35 mg/kg (mouse, i.p.); 56 mg/kg (mouse, i.v.)[citation needed]
See also
editReferences
edit- ^ The Merck Index, 10th Ed., p.1316, Rahway: Merck & Co.
- ^ A. A. Vernon and J. L. Sheard (1948). "The solubility of tetraethylammonium iodide in benzene-ethylene dichloride mixtures." J. Am. Chem. Soc. 70 2035-2036. https://doi.org/10.1021/ja01186a015
- ^ E. Wait and H. M. Powell (1958). "The crystal and molecular structure of tetraethylammonium iodide." J. Chem. Soc. 1872-1875.
- ^ N. Hénaff and A. Whiting (2000). "Stereoselective formation of 1,2-diiodoalkenes and their application in the stereoselective synthesis of highly functionalised alkenes via Suzuki and Stille coupling reactions." J. Chem. Soc., Perkin 1 395-400.
- ^ T.Yoshino et al. (1977). "Synthetic studies with carbonates. Part 6. Syntheses of 2-hydroxyethyl derivatives by reactions of ethylene carbonate with carboxylic acids or heterocycles in the presence of tetraethylammonium halides or under autocatalytic conditions." J. Chem. Soc., Perkin 1 1266-1272.
- ^ G. Saikia and P. K. Iyer (2010)."Facile C-H alkylation in water: enabling defect-free materials for optoelectronic devices." J. Org. Chem. 75 2714-2717.