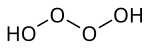
Tetraoxidane is an inorganic compound of hydrogen and oxygen with the chemical formula H
2O
4.[1][2][3] This is one of the unstable hydrogen polyoxides.[4]
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Tetraoxidane
| |||
| Other names
Hydroxyperoxide, dihydrogen tetroxide, diperoxide, bisperoxide
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
PubChem CID
|
|||
| |||
| |||
| Properties | |||
| H2O4 | |||
| Molar mass | 66.012 g·mol−1 | ||
| Density | 1.8±0.1 g/cm3 | ||
| Related compounds | |||
Related compounds
|
Pentaoxidane | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Synthesis
editThe compound is prepared by a chemical reaction between hydroperoxyl radicals (HO2•) at low temperatures:[5][6]
Physical properties
editThis is the fourth member of the polyoxidanes. The first three are water [(mon)oxidane], hydrogen peroxide (dioxidane), and trioxidane. Tetroxidane is more unstable than the previous compounds. The term "tetraoxidane" extends beyond the parent compound to several daughter compounds of the general formula R
2O
4, where R can be hydrogen, halogen atoms, or various inorganic and organic monovalent radicals. The two Rs together can be replaced by a divalent radical, so heterocyclic tetroxidanes also exist.[7]
Ionization
editTetroxidane autoionizes when in liquid form:
References
edit- ^ Mckay, Daniel J.; Wright, James S. (1 February 1998). "How Long Can You Make an Oxygen Chain?". Journal of the American Chemical Society. 120 (5): 1003–1013. doi:10.1021/ja971534b. ISSN 0002-7863. Retrieved 16 May 2023.
- ^ "hydroxyperoxide". ChemScr. Retrieved 15 May 2023.
- ^ The Chemistry of Peroxides, Volume 3. John Wiley & Sons. 20 April 2015. p. 198. ISBN 978-1-118-41271-8. Retrieved 15 May 2023.
- ^ "Selected ATcT [1, 2] enthalpy of formation based on version 1.122 of the Thermochemical Network [3]". atct.anl.gov. Retrieved 15 May 2023.
- ^ Levanov, Alexander V.; Sakharov, Dmitri V.; Dashkova, Anna V.; Antipenko, Ewald E.; Lunin, Valeri V. (2011). "Synthesis of Hydrogen Polyoxides H2O4 and H2O3 and Their Characterization by Raman Spectroscopy". European Journal of Inorganic Chemistry. 2011 (33): 5144–5150. doi:10.1002/ejic.201100767.
- ^ Möller, Detlev (19 February 2019). Fundamentals and Processes. Walter de Gruyter GmbH & Co KG. p. 276. ISBN 978-3-11-056126-5. Retrieved 15 May 2023.
- ^ Curutchet, Antton; Colinet, Pauline; Michel, Carine; Steinmann, Stephan N.; Le Bahers, Tangui (2020). "Two-sites are better than one: revisiting the OER mechanism on CoOOH by DFT with electrode polarization" (PDF). Physical Chemistry Chemical Physics. 22 (13): 7031–7038. Bibcode:2020PCCP...22.7031C. doi:10.1039/D0CP00281J. PMID 32195492. S2CID 213191538. Retrieved 15 May 2023.