Pyruvate dehydrogenase is an enzyme that catalyzes the reaction of pyruvate and a lipoamide to give the acetylated dihydrolipoamide and carbon dioxide. The conversion requires the coenzyme thiamine pyrophosphate.
| pyruvate dehydrogenase (acetyl-transferring) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
 Crystallographic structure of pyruvate dehydrogenase (PDH). PH is a six domain dimer with α (blue), α’ (yellow), β (red), and β’ (teal) regions denoted by the different colors. Thiamine pyrophosphate (TPP) is shown in grey ball and stick form, two magnesium ions in purple undergoing metal ligation with the TPP, and two potassium ions in orange.[1] | |||||||||
| Identifiers | |||||||||
| EC no. | 1.2.4.1 | ||||||||
| CAS no. | 9014-20-4 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| Gene Ontology | AmiGO / QuickGO | ||||||||
| |||||||||
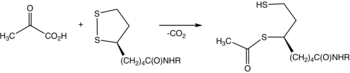
Pyruvate dehydrogenase is usually encountered as a component, referred to as E1, of the pyruvate dehydrogenase complex (PDC). PDC consists of other enzymes, referred to as E2 and E3. Collectively E1-E3 transform pyruvate, NAD+, coenzyme A into acetyl-CoA, CO2, and NADH. The conversion is crucial because acetyl-CoA may then be used in the citric acid cycle to carry out cellular respiration.[2] To distinguish between this enzyme and the PDC, it is systematically called pyruvate dehydrogenase (acetyl-transferring).
Mechanism
editThe thiamine pyrophosphate (TPP) converts to an ylide by deprotonation. The ylide attack the ketone group of pyruvate. The resulting adduct decarboxylates. The resulting 1,3-dipole reductively acetylates lipoamide-E2.[2]
In terms of details, biochemical and structural data for E1 revealed a mechanism of activation of TPP coenzyme by forming the conserved hydrogen bond with glutamate residue (Glu59 in human E1) and by imposing a V-conformation that brings the N4’ atom of the aminopyrimidine to intramolecular hydrogen bonding with the thiazolium C2 atom. This unique combination of contacts and conformations of TPP leads to formation of the reactive C2-carbanion, eventually. After the cofactor TPP decarboxylates pyruvate, the acetyl portion becomes a hydroxyethyl derivative covalently attached to TPP.[1]
Structure
editE1 is a multimeric protein. Mammalian E1s, including human E1, are tetrameric, composed of two α- and two β- subunits.[1] Some bacterial E1s, including E1 from Escherichia coli, are composed of two similar subunits, each being as large as the sum of molecular masses of α- and β- subunits.[3]
Active site
editE1 has two catalytic sites, each providing thiamine pyrophosphate (TPP) and magnesium ion as cofactors. The α- subunit binds magnesium ion and pyrophosphate fragment while the β-subunit binds pyrimidine fragment of TPP, forming together a catalytic site at the interface of subunits.[1]
The active site for pyruvate dehydrogenase (image created from PDB: 1NI4) holds TPP through metal ligation to a magnesium ion (purple sphere) and through hydrogen bonding to amino acids. While over 20 amino acids can be found in the active site, amino acids Tyr 89, Arg 90, Gly 136, Val 138, Asp 167, Gly 168, Ala 169, Asn, 196, and His 263 actually participate in hydrogen bonding to hold TPP and pyruvate (not shown here) in the active site. The amino acids are shown as wires, and the TPP is in ball and stick form. The active site also aids in the transfer of the acyl on the TPP to a lipoamide waiting on E2.[1]
Regulation
editPhosphorylation of E1 by pyruvate dehydrogenase kinase (PDK) inactivates E1 and subsequently the entire complex. PDK is inhibited by dichloroacetic acid and pyruvate, resulting in a higher quantity of active, unphosphorylated PDH.[4] Phosphorylation is reversed by pyruvate dehydrogenase phosphatase, which is stimulated by insulin, PEP, and AMP, but competitively inhibited by ATP, NADH, and Acetyl-CoA.
Pathology
editPyruvate dehydrogenase is targeted by an autoantigen known as anti-mitochondrial antibodies (AMA), which results in progressive destruction of the small bile ducts of the liver, leading to primary biliary cirrhosis. These antibodies appear to recognize oxidized protein that has resulted from inflammatory immune responses. Some of these inflammatory responses could be related to gluten sensitivity as over 50% of the acute liver failure patients in one study exhibited a nonmitochondrial autoantibody against tissue transglutaminase.[5] Other mitochondrial autoantigens include oxoglutarate dehydrogenase and branched-chain alpha-keto acid dehydrogenase complex, which are antigens recognized by anti-mitochondrial antibodies.
Increased pyruvate dehydrogenase (PDH) activity can cause oncogene-induced cellular senescence, as well as promoting aging.[6] Decreased activity of mitochondrial PDH with age has been shown in the heart as well as in certain regions of the brain (the striatum and brainstem).[6]
Pyruvate dehydrogenase (PDH) deficiency is a congenital degenerative metabolic disease resulting from a mutation of the pyruvate dehydrogenase complex (PDC) located on the X chromosome. While defects have been identified in all 3 enzymes of the complex, the E1-α subunit is predominantly the culprit. Malfunction of the citric acid cycle due to PDH deficiency deprives the body of energy and leads to an abnormal buildup of lactate. PDH deficiency is a common cause of lactic acidosis in newborns and often presents with severe lethargy, poor feeding, tachypnea, and cases of death have occurred.[7]
Examples
editHuman proteins that possess pyruvate dehydrogenase activity include:
|
|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Related enzymes
editIn bacteria, a form of pyruvate dehydrogenase (also called pyruvate oxidase, EC 1.2.2.2) exists that links the oxidation of pyruvate into acetate and carbon dioxide to the reduction of ferrocytochrome. In E. coli this enzyme is encoded by the pox B gene and the protein has a flavin cofactor.[8] This enzyme increases the efficiency of growth of E. coli under aerobic conditions.[9]
See also
editReferences
edit- ^ a b c d e PDB: 1ni4; Ciszak EM, Korotchkina LG, Dominiak PM, Sidhu S, Patel MS (June 2003). "Structural basis for flip-flop action of thiamin pyrophosphate-dependent enzymes revealed by human pyruvate dehydrogenase". J. Biol. Chem. 278 (23): 21240–6. doi:10.1074/jbc.M300339200. hdl:2060/20030106063. PMID 12651851.
- ^ a b J. M. Berg; J. L. Tymoczko, L. Stryer (2007). Biochemistry (6th ed.). Freeman. ISBN 978-0-7167-8724-2.
- ^ Arjunan P, Nemeria N, Brunskill A, Chandrasekhar K, Sax M, Yan Y, et al. (April 2002). "Structure of the pyruvate dehydrogenase multienzyme complex E1 component from Escherichia coli at 1.85 A resolution". Biochemistry. 41 (16): 5213–21. doi:10.1021/bi0118557. PMID 11955070.
- ^ Jaimes, R 3rd (Jul 2015). "Functional response of the isolated, perfused normoxic heart to pyruvate dehydrogenase activation by dichloroacetate and pyruvate". Pflügers Arch. 468 (1): 131–42. doi:10.1007/s00424-015-1717-1. PMC 4701640. PMID 26142699.
{{cite journal}}: CS1 maint: numeric names: authors list (link) - ^ Leung PS, Rossaro L, Davis PA, et al. (2007). "Antimitochondrial antibodies in acute liver failure: Implications for primary biliary cirrhosis". Hepatology. 46 (5): 1436–42. doi:10.1002/hep.21828. PMC 3731127. PMID 17657817.
- ^ a b Veech RL, Bradshaw PC, King MT (2017). "Ketone bodies mimic the life span extending properties of caloric restriction". IUBMB Life. 69 (5): 305–314. doi:10.1002/iub.1627. PMID 28371201. S2CID 19807849.
- ^ Pyruvate Dehydrogenase Complex Deficiency at eMedicine
- ^ Recny MA, Hager LP (1982). "Reconstitution of native Escherichia coli pyruvate oxidase from apoenzyme monomers and FAD". J. Biol. Chem. 257 (21): 12878–86. doi:10.1016/S0021-9258(18)33597-X. PMID 6752142.
- ^ Abdel-Hamid AM, Attwood MM, Guest JR (2001). "Pyruvate oxidase contributes to the aerobic growth efficiency of Escherichia coli". Microbiology. 147 (Pt 6): 1483–98. doi:10.1099/00221287-147-6-1483. PMID 11390679.
- Ochoa S (1954). "Enzymic Mechanisms in the Citric Acid Cycle". Advances in Enzymology and Related Areas of Molecular Biology. Advances in Enzymology - and Related Areas of Molecular Biology. Vol. 15. pp. 183–270. doi:10.1002/9780470122600.ch5. ISBN 9780470122600. PMID 13158180.
{{cite book}}:|journal=ignored (help) - Scriba P, Holzer H (1961). "Gewinnung von alphaHydroxyathyl-2-thiaminpyrophosphat mit Pyruvatoxydase aus Schweineherzmuskel". Biochem. Z. 334: 473–486.
- Perham RN (2000). "Swinging arms and swinging domains in multifunctional enzymes: catalytic machines for multistep reactions". Annual Review of Biochemistry. 69 (1): 961–1004. doi:10.1146/annurev.biochem.69.1.961. PMID 10966480.
External links
edit- Pyruvate+Dehydrogenase-E1 at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
- http://www.brookscole.com/chemistry_d/templates/student_resources/shared_resources/animations/pdc/pdc.html Archived 2012-07-24 at the Wayback Machine
- PDBe-KB provides an overview of all the structure information available in the PDB for Human Pyruvate dehydrogenase (lipoamide) alpha 1.
- PDBe-KB provides an overview of all the structure information available in the PDB for Human pyruvate dehydrogenase (lipoamide) beta.